In the last few posts, I have explored the anomeric effect as it occurs at an atom centre X. Here I try to summarise the atoms for which the effect is manifest in crystal structures.
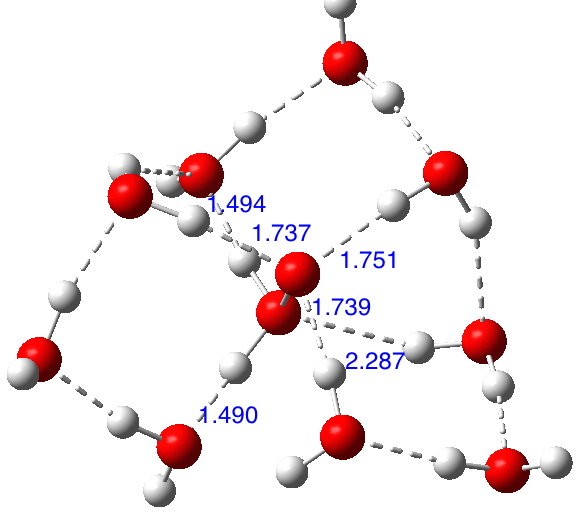
Here is a little molecule that can be said to be pretty electron rich. There are lots of lone pairs present, and not a few electron-deficient σ-bonds. I thought it might be fun to look at the stereoelectronic interactions set up in this little system.
The autoionization of water involves two molecules transfering a proton to give hydronium hydroxide, a process for which the free energy of reaction is well known. Here I ask what might happen with the next element along in the periodic table, F. I have been unable to find much about the autoionization of HF in the literature;
Earlier, I constructed a possible model of hydronium hydroxide, or H3O+.OH– One way of assessing the quality of the model is to calculate the free energy difference between it and two normal water molecules and compare the result to the measured difference. Here I apply a further test of the model using isotopes.
If H3N+-O– is viable compared with its tautomer H2N-OH when carrying water bridges, then why not try H2O+-O– vs HO-OH? https://orcid.org/0000-0002-8635-8390
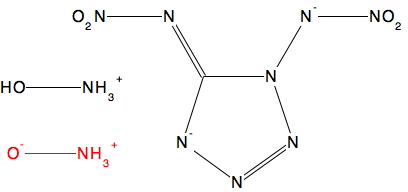
In the previous post I described how hydronium hydroxide or H3O+…HO–, an intermolecular tautomer of water, has recently been observed captured inside an organic cage[cite]10.1002/chem.201406383[/cite] and how the free-standing species in water can be captured computationally with the help of solvating water bridges.
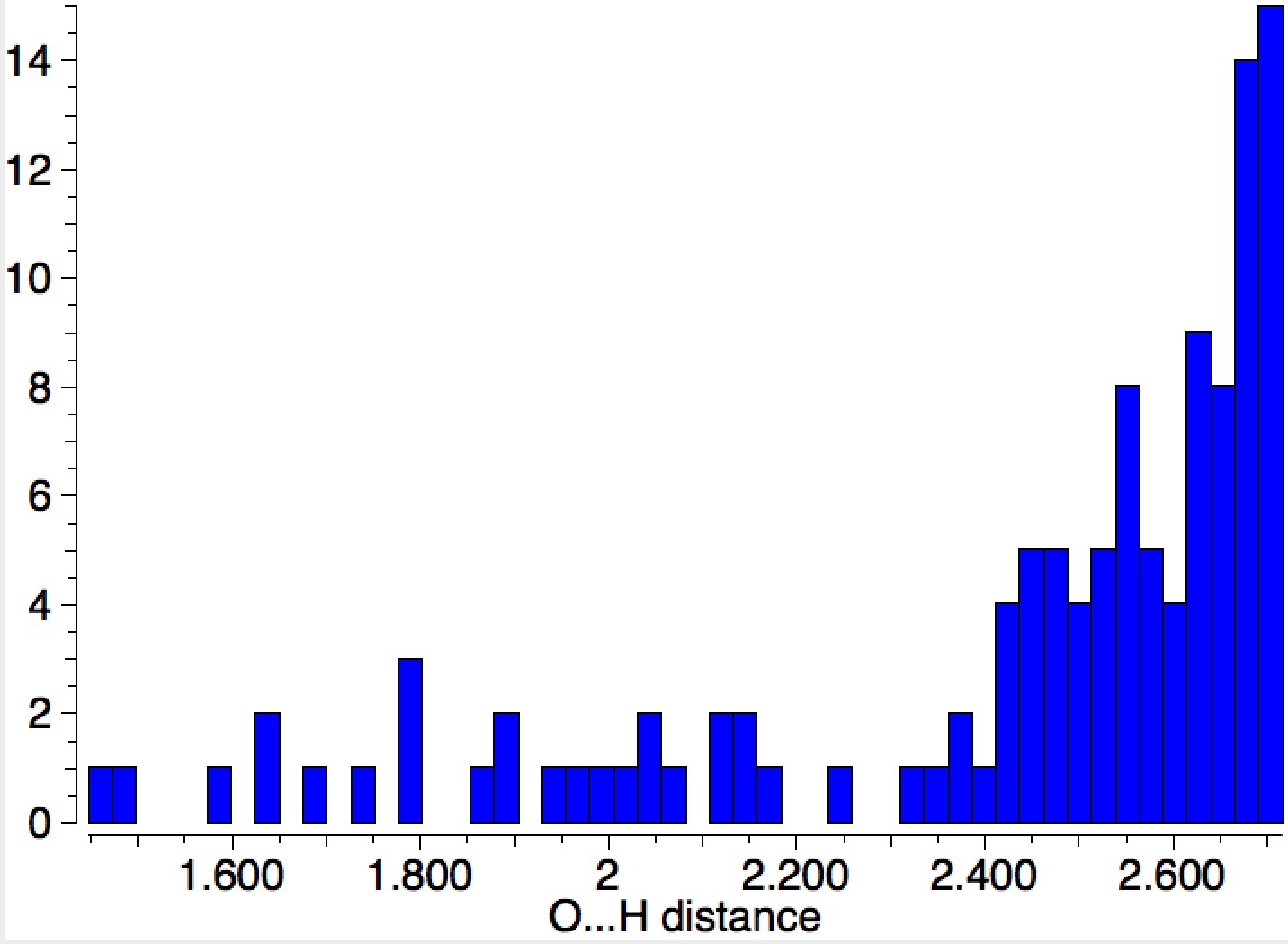
Ammonium hydroxide (NH4+…OH–) can be characterised quantum mechanically when stabilised by water bridges connecting the ion-pairs.
Previously, I looked at models of how ammonia could be protonated by water to form ammonium hydroxide.
A celebration of the life and work of the great chemist Paul von R. Schleyer was held this week in Erlangen, Germany. There were many fantastic talks given by some great chemists describing fascinating chemistry. Here I highlight the presentation given by Andy Streitwieser on the topic of organolithium chemistry, also a great interest of Schleyer's over the years.
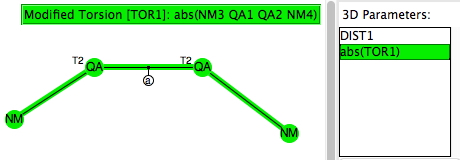
The upcoming ACS national meeting in San Diego has a CHED (chemical education division) session entitled Implementing Discovery-Based Research Experiences in Undergraduate Chemistry Courses. I had previously explored what I called extreme gauche effects in the molecule F-S-S-F. Here I take this a bit further to see what else can be discovered about molecules containing bonds between group 16 elements (QA= O, S, Se, Te).