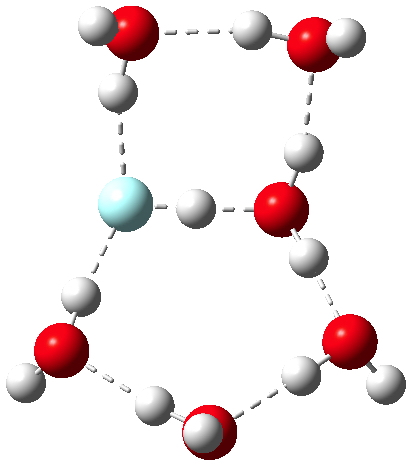
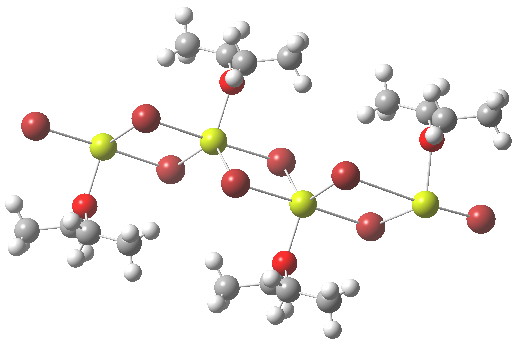
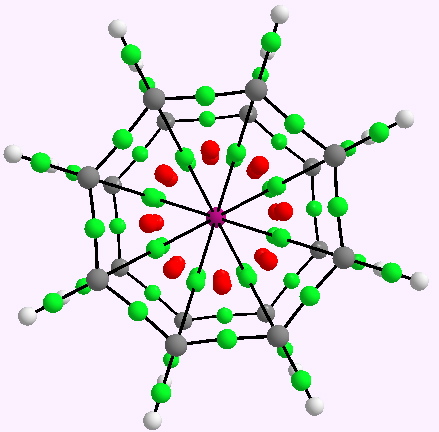
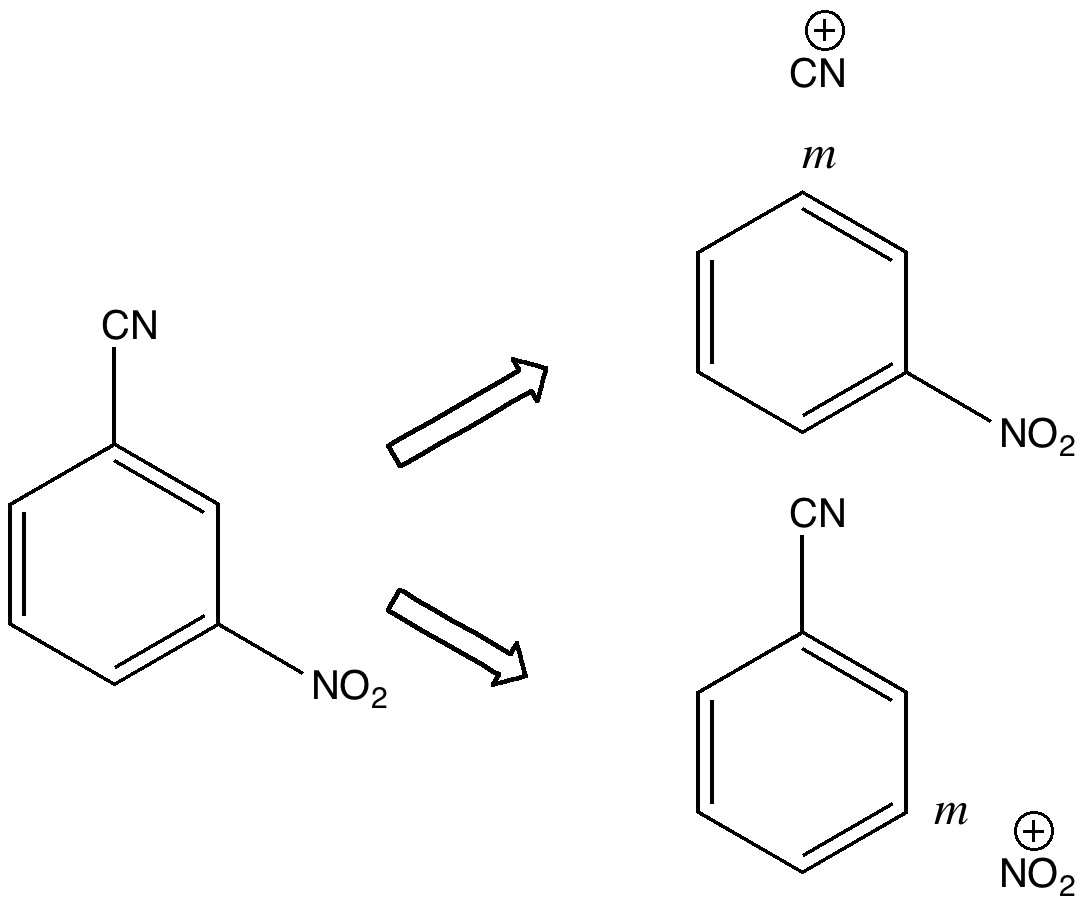
My previous posts have covered the ionization by a small number of discrete water molecules of the series of halogen acids, ranging from HI (the strongest, pKa -10) via HF (weaker, pKa 3.1) to the pseudo-halogen HCN (the weakest, pKa 9.2). Here I try out some even stronger acids to see what the least number of water molecule needed to ionize these might be. Firstly what must surely be the ultimate acid