Previously, I explored the Graham reaction to form a diazirine. The second phase of the reaction involved an Sn2′ displacement of N-Cl forming C-Cl. Here I ask how facile the simpler displacement of C-Cl by another chlorine might be and whether the mechanism is Sn2 or the alternative Sn1.
Symbiosis between computation and experiment is increasingly evident in pedagogic journals such as J. Chemical Education . Thus an example of original laboratory experiments[cite]10.1021/ed077p271[/cite],[cite]10.1021/ed078p1266[/cite] that later became twinned with a computational counterpart.[cite]10.1021/ed500398e[/cite] So when I spotted this recent lab experiment[cite]10.1021/acs.jchemed.7b00566[/cite] I felt another twinning
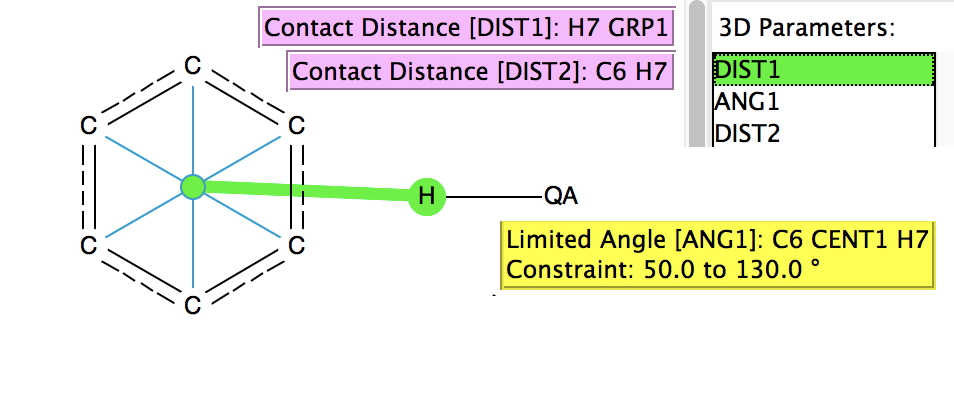
A few years back, I did a post about the Pirkle reagent[cite]10.1039/c39910000765[/cite] and the unusual π-facial hydrogen bonding structure[cite]10.1039/P29940000703[/cite] it exhibits. For the Pirkle reagent, this bonding manifests as a close contact between the acidic OH hydrogen and the edge of a phenyl ring; the hydrogen bond is off-centre from the middle of the aryl ring.
Ammonium hydroxide (NH4+…OH–) can be characterised quantum mechanically when stabilised by water bridges connecting the ion-pairs.

I recently followed this bloggers trail; link1 → link2 to arrive at this delightful short commentary on atom-atom bonds in crystals[cite]10.1107/S2052252515002006[/cite] by Jack Dunitz. Here he discusses that age-old question (to chemists), what is a bond? Even almost 100 years after Gilbert Lewis’ famous analysis,[cite]10.1021/ja02261a002[/cite] we continue to ponder this question.
