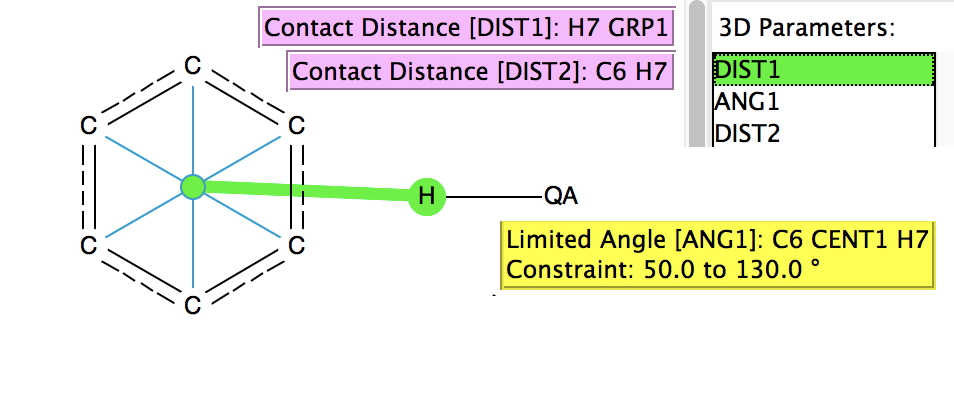
A few years back, I did a post about the Pirkle reagent[cite]10.1039/c39910000765[/cite] and the unusual π-facial hydrogen bonding structure[cite]10.1039/P29940000703[/cite] it exhibits. For the Pirkle reagent, this bonding manifests as a close contact between the acidic OH hydrogen and the edge of a phenyl ring; the hydrogen bond is off-centre from the middle of the aryl ring.