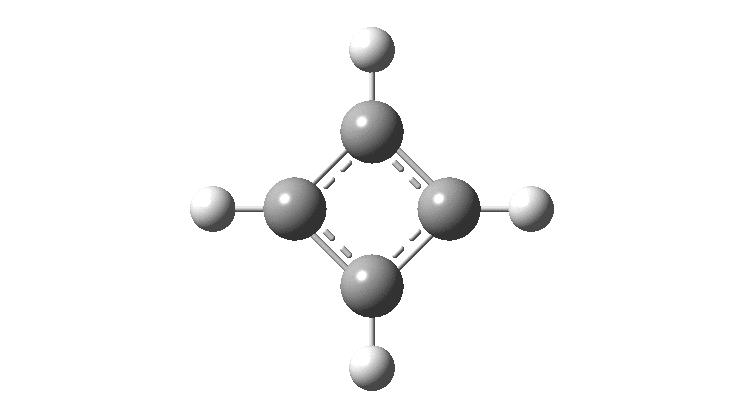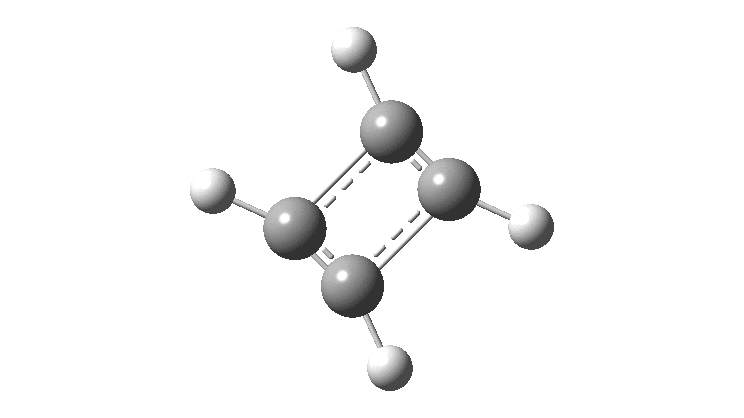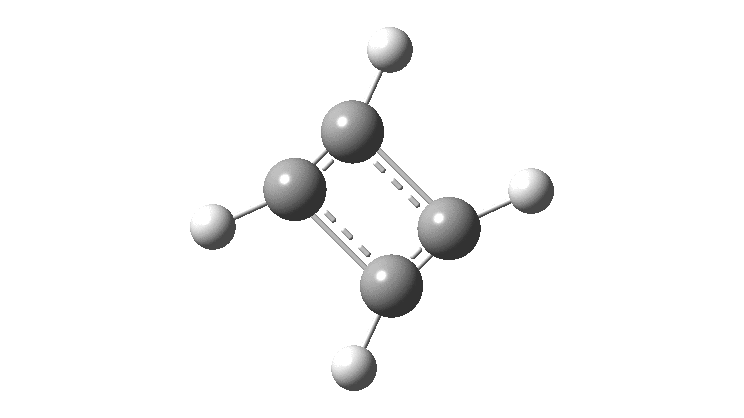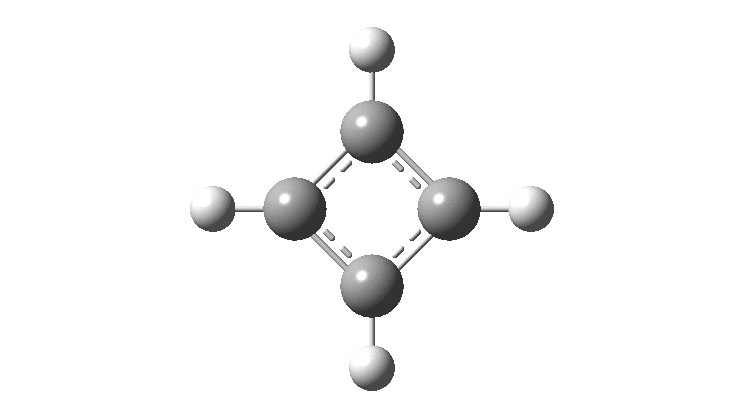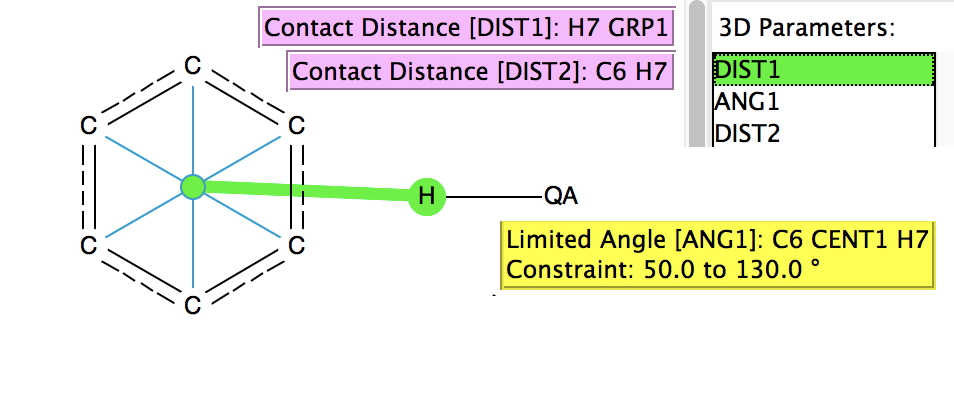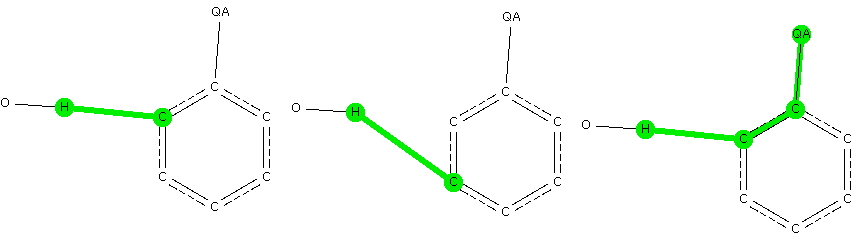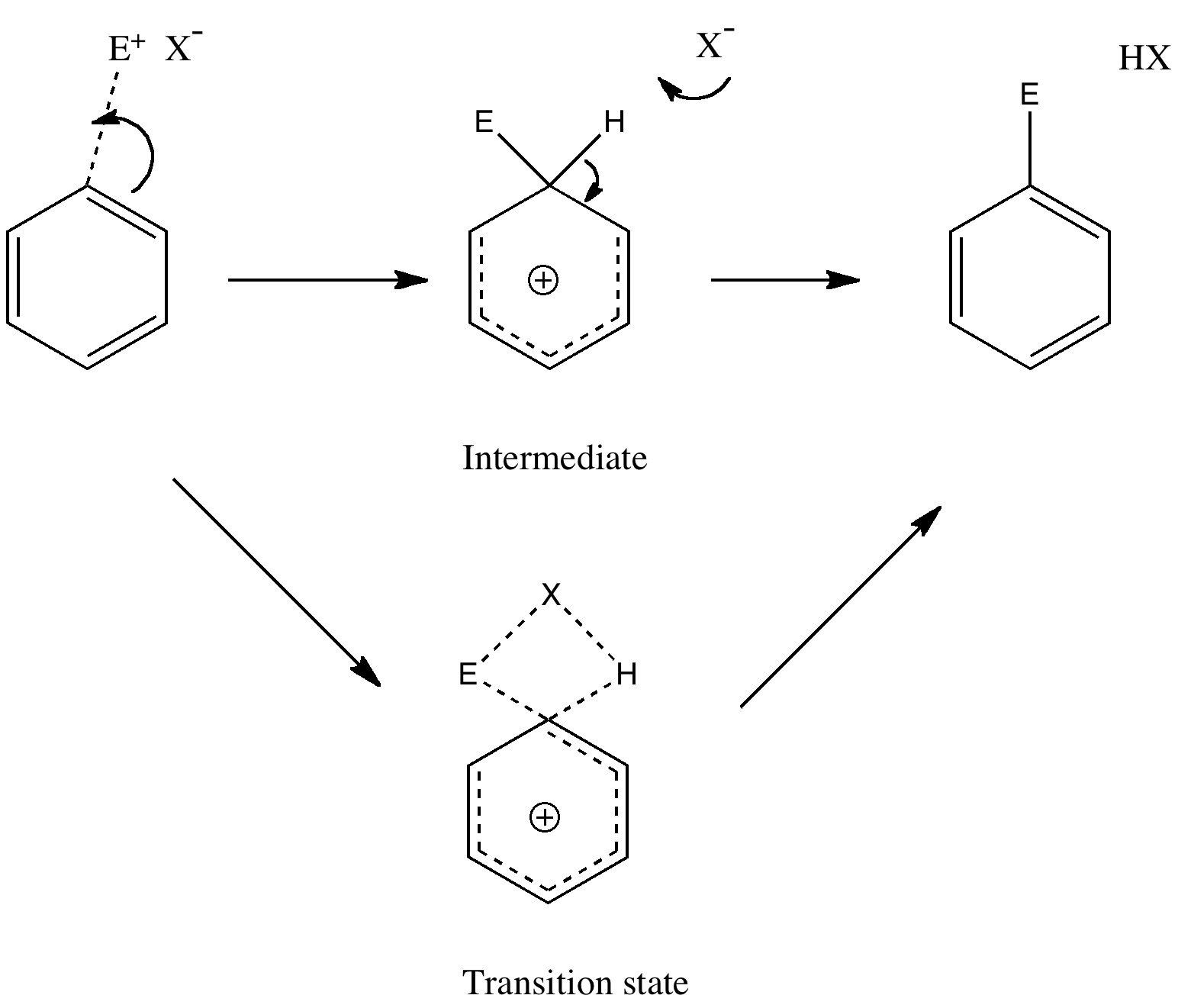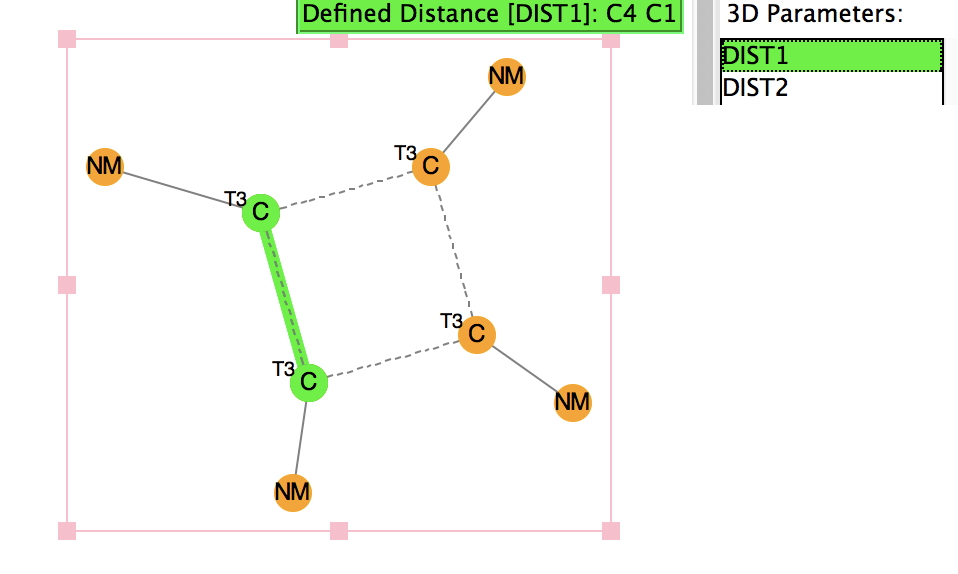
There is emerging interest in cyclic conjugated molecules that happen to have triplet spin states and which might be expected to follow a 4n rule for aromaticity.[cite]10.1002/anie.201705228[/cite] The simplest such system would be the triplet state of cyclobutadiene, for which a non or anti-aromatic singlet state is always found to be lower in energy. Here I explore some crystal structures containing this motif for possible insights.