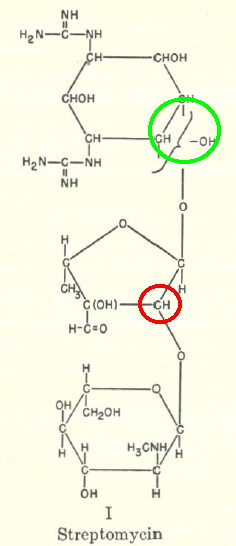
Streptomycin is an antibiotic active against tuberculosis, and its discovery has become something of a cause célèbre. It was first isolated on October 19, 1943 by a graduate student Albert Schatz in the laboratory of Selman Waksman at Rutgers University. I want to concentrate in this post on its molecular structure.