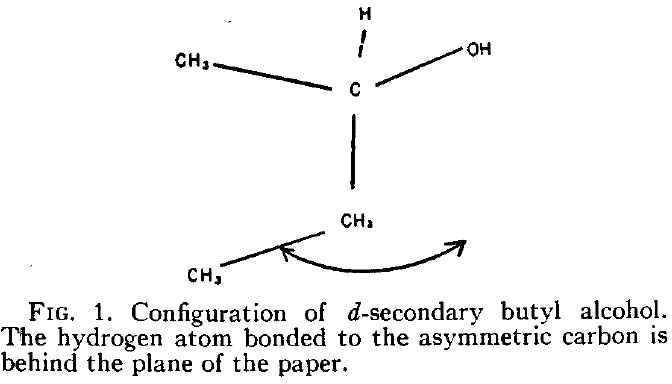
Some areas of science progressed via very famous predictions that were subsequently verified by experiments. Think of Einstein and gravitational waves or of Dirac and the positron. There are fewer well-known examples in chemistry; perhaps Watson and Crick’s prediction of the structure of DNA, albeit based on the interpretation of an existing experimental result.