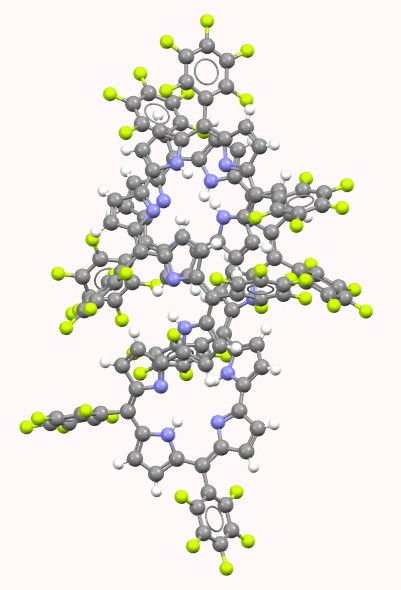
My last comment as appended to the previous post promised to analyse two so-called extended porphyrins for their topological descriptors. I start with the Cãlugãreanu/Fuller theorem

My last comment as appended to the previous post promised to analyse two so-called extended porphyrins for their topological descriptors. I start with the Cãlugãreanu/Fuller theorem
AbstractA series of meso‐trifluoromethyl‐substituted expanded porphyrins, including N‐fused [24]pentaphyrin 3, [28]hexaphyrin 4, [32]heptaphyrin 5, [46]decaphyrin 6, and [56]dodecaphyrin 7, were synthesized by means of an acid‐catalyzed one‐pot condensation reaction of 2‐(2,2,2‐trifluoro‐1‐hydroxyethyl)pyrrole (1) as the first examples bearing meso‐alkyl substituents. Besides these products, porphyrin 2 and two calix[5]phyrins 8 and 9 were also obtained. [28]Hexaphyrin 4 was quantitatively oxidized to [26]hexaphyrin 14 with MnO2. These expanded porphyrins have been characterized by mass spectrometry, 1H and 19F NMR spectroscopy, and UV/Vis spectroscopy. The single‐crystal structures have been determined for 3, 4, 6, 7, and 14. The N‐fused [24]pentaphyrin 3 displays a distorted structure containing a tricyclic fused moiety that is similar to those of meso‐aryl‐substituted counterparts, whereas 8 and 9 are indicated to take roughly planar conformations with an inverted pyrrole opposite to the sp3‐hybridized meso‐carbon atom. Both [28]‐ and [26]hexaphyrins 4 and 14 have figure‐of‐eight structures. Solid‐state structures of the decaphyrin 6 and dodecaphyrin 7 are remarkable, exhibiting a crescent conformation and an intramolecular two‐pitch helical conformation, respectively.
Abstractmeso‐Aryl substituted rubyrin ([26]hexaphyrin(1.1.0.1.1.0)) 2 and a series of rubyrin‐type large expanded porphyrins were obtained from a facile one‐pot oxidative coupling reaction of meso‐pentafluorophenyl substituted tripyrrane 1. The structures of two of the resulting products were determined by single‐crystal X‐ray diffraction analysis. Whereas [52]dodecaphyrin(1.1.0.1.1.0.1.1.0.1.1.0) 4 takes a symmetric helical conformation, the larger species, [62]pentadecaphyrin(1.1.0.1.1.0.1.1.0.1.1.0.1.1.0) 5, adopts a nonsymmetric distorted conformation in the solid state that contains an intramolecular helical structure. The ability of rubyrin 2 to act as an anion receptor in its diprotonated form (2⋅2H+) was demonstrated in methanolic solutions. Oxidation of 2 with MnO2 gave [24]rubyrin 6, a species that displays antiaromatic characteristics. [26]Rubyrin 2 and [24]rubyrin 6 both underwent metallation when reacted with Zn(OAc)2 to give the corresponding bis‐zinc(II) complexes 7 and 8 quantitatively without engendering a change in the oxidation state of the ligands. As a result, complexes 7 and 8 exhibit aromatic and antiaromatic character, respectively. NICS calculation on these compounds also supported aromaticity of 2 and 7, and antiaromaticity of 6 and 8.