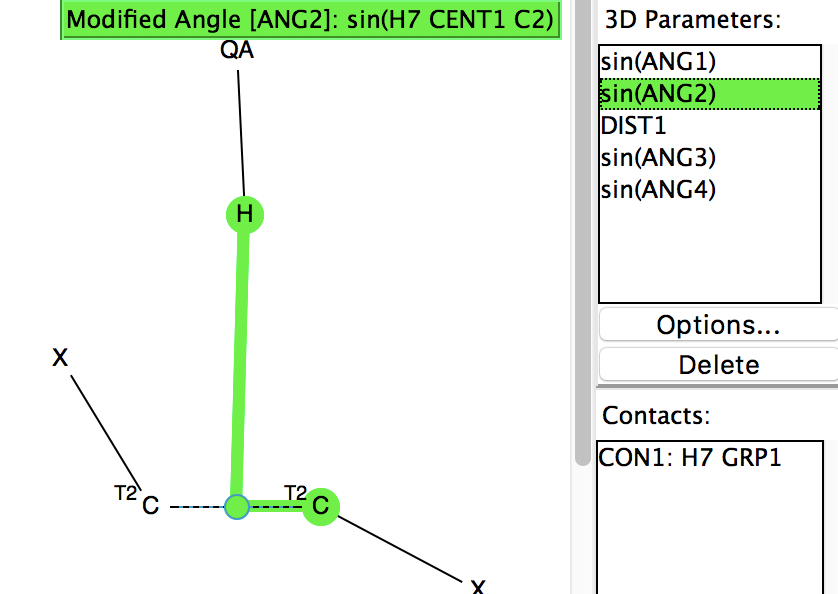
Following on from my re-investigation of close hydrogen bonding contacts to the π-face of alkenes, here now is an updated scan for H-bonds to alkynes. The search query (dataDOI: 10.14469/hpc/2478) is similar to the previous one: QA is any of N,O,F,Cl. X is any atom, including metals and non-metals.