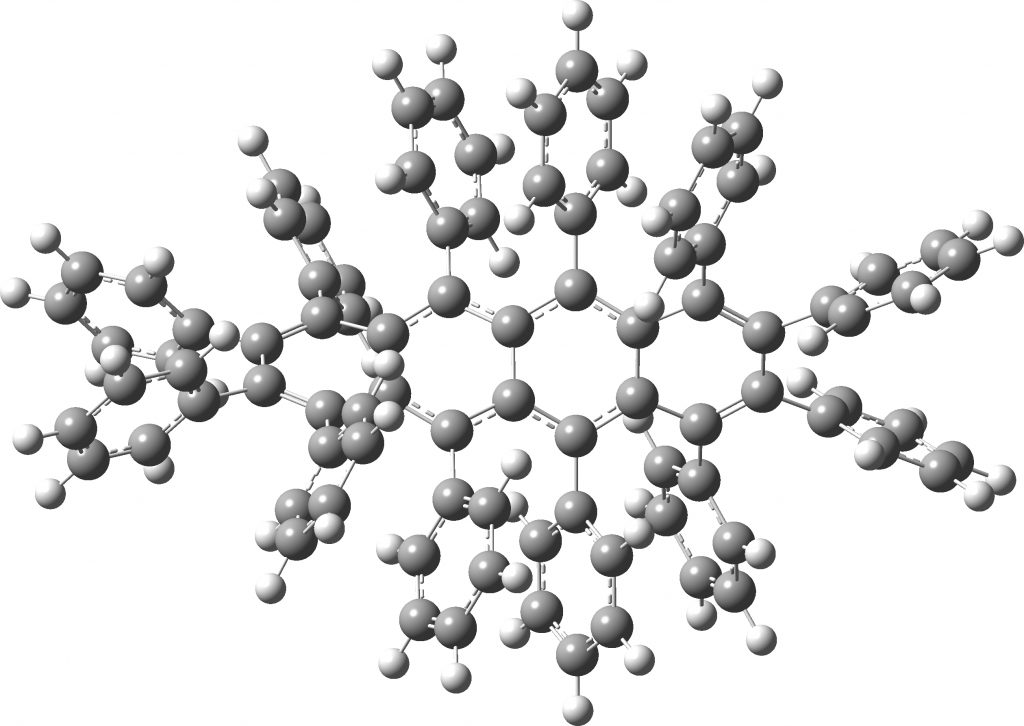
All of the molecules in this year’s C&EN list are fascinating in their very different ways. Here I take a look at the twisty tetracene (dodecaphenyltetracene) which is indeed very very twisty.[cite]10.1002/anie.201812418[/cite] Click on image to view 3D model Unfortunately, the authors point that the twisty-ness does not lead to a stable helical configuration at room temperatures and so separate enantiomers cannot be isolated.