The Science education unit at the ACS publication C&EN publishes its list of molecules of the year (as selected by the editors and voted upon by the readers) in December. Here are some observations about three of this year’s batch.

The Science education unit at the ACS publication C&EN publishes its list of molecules of the year (as selected by the editors and voted upon by the readers) in December. Here are some observations about three of this year’s batch.
AbstractThe control of tetrahedral carbon stereocentres remains a focus of modern synthetic chemistry and is enabled by their configurational stability. By contrast, trisubstituted nitrogen1, phosphorus2 and sulfur compounds3 undergo pyramidal inversion, a fundamental and well-recognized stereochemical phenomenon that is widely exploited4. However, the stereochemistry of oxonium ions—compounds bearing three substituents on a positively charged oxygen atom—is poorly developed and there are few applications of oxonium ions in synthesis beyond their existence as reactive intermediates5,6. There are no examples of configurationally stable oxonium ions in which the oxygen atom is the sole stereogenic centre, probably owing to the low barrier to oxygen pyramidal inversion7 and the perception that all oxonium ions are highly reactive. Here we describe the design, synthesis and characterization of a helically chiral triaryloxonium ion in which inversion of the oxygen lone pair is prevented through geometric restriction to enable it to function as a determinant of configuration. A combined synthesis and quantum calculation approach delineates design principles that enable configurationally stable and room-temperature isolable salts to be generated. We show that the barrier to inversion is greater than 110 kJ mol−1 and outline processes for resolution. This constitutes, to our knowledge, the only example of a chiral non-racemic and configurationally stable molecule in which the oxygen atom is the sole stereogenic centre.
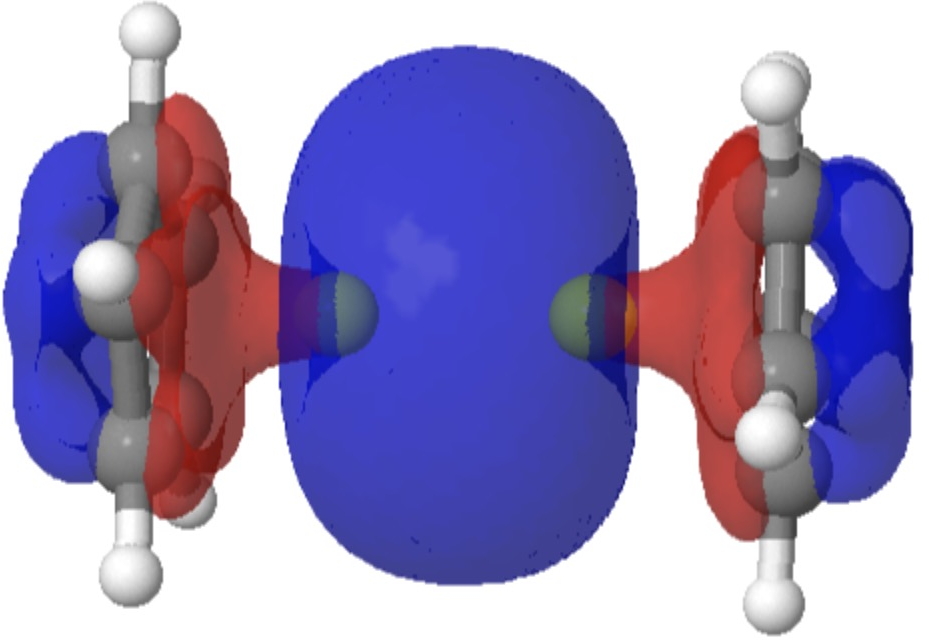
The complex diberyllocene, CpBeBeCp (Cp, cyclopentadienyl anion), has been the subject of numerous chemical investigations over the past five decades yet has eluded experimental characterization. We report the preparation and isolation of the compound by the reduction of beryllocene (BeCp 2 ) with a dimeric magnesium(I) complex and determination of its structure in the solid state by means of x-ray crystallography. Diberyllocene acts as a reductant in reactions that form beryllium-aluminum and beryllium-zinc bonds. Quantum chemical calculations indicate parallels between the electronic structure of diberyllocene and the simple homodiatomic species diberyllium (Be 2 ).
Sometimes, the properties of a molecule are predicted long before it is synthesised. One such is diberyllocene. I first encountered a related molecule, beryllocene itself, many moons ago.[1] This was unusual because unlike the original metallocenes, the metal atom was not symmetrically disposed between the two cyclopentadienyl faces.