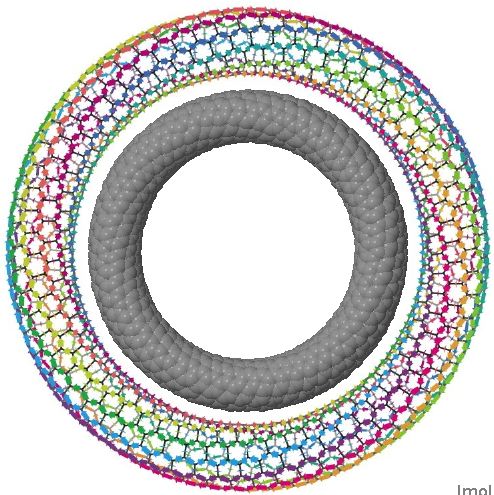
The interface between physics, chemistry (and materials science) can be a fascinating one. Here I show a carbon nanotorus, devised by physicists[cite]10.1103/PhysRevLett.88.217206[/cite] a few years ago. It is a theoretical species, and was predicted to have a colossal paramagnetic moment . Carbon nanotorus.