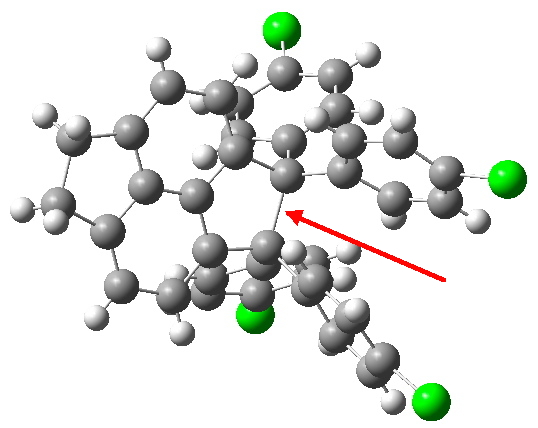
Here is a challenge: what is the longest C-C bond actually determined (in which both carbon termini are sp 3 hybridised)? I pose this question since Steve Bachrach has posted on how to stabilize long bonds by attractive dispersive interactions, and more recently commenting on what the longest straight chain alkane might be before dispersive interaction start to fold it (the answer appears to be C 17 ). A search of the