Following on from Armstrong’s almost electronic theory of chemistry in 1887-1890, and Beckmann’s radical idea around the same time that molecules undergoing transformations might do so via a reaction mechanism involving unseen intermediates (in his case, a transient enol of a ketone) I here describe how these concepts underwent further evolution in the early 1920s.
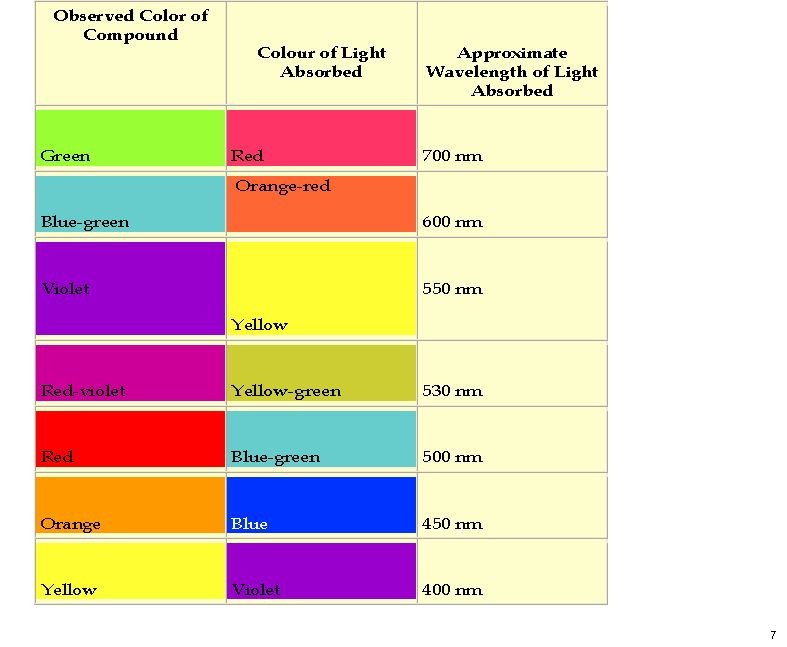
My previous post on the topic of mauveine left the outcome dangling. Put simply, λ max is measured at about 549nm for mauveine A, but was calculated at about 440nm using a modern method for predicting colour (TD-DFT). According to the colour table below, that would make it orange, not mauve. Can the theoretical prediction be out by 110nm, or might it be the structure of the molecule itself that has been wrongly described?
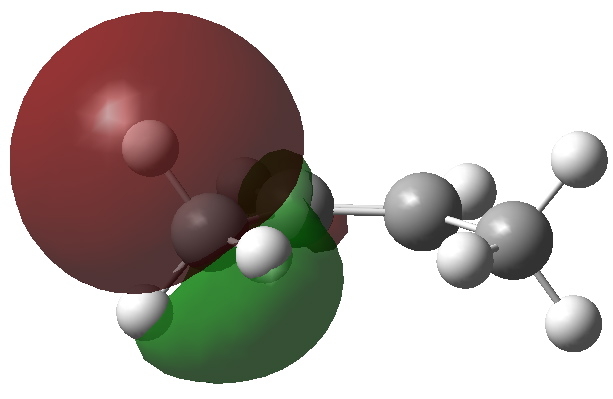
I wrote earlier about the strangely close contact between two hydrogen atoms in cis -butene. The topology of the electron density showed characteristics of a bond, but is it a consensual union?
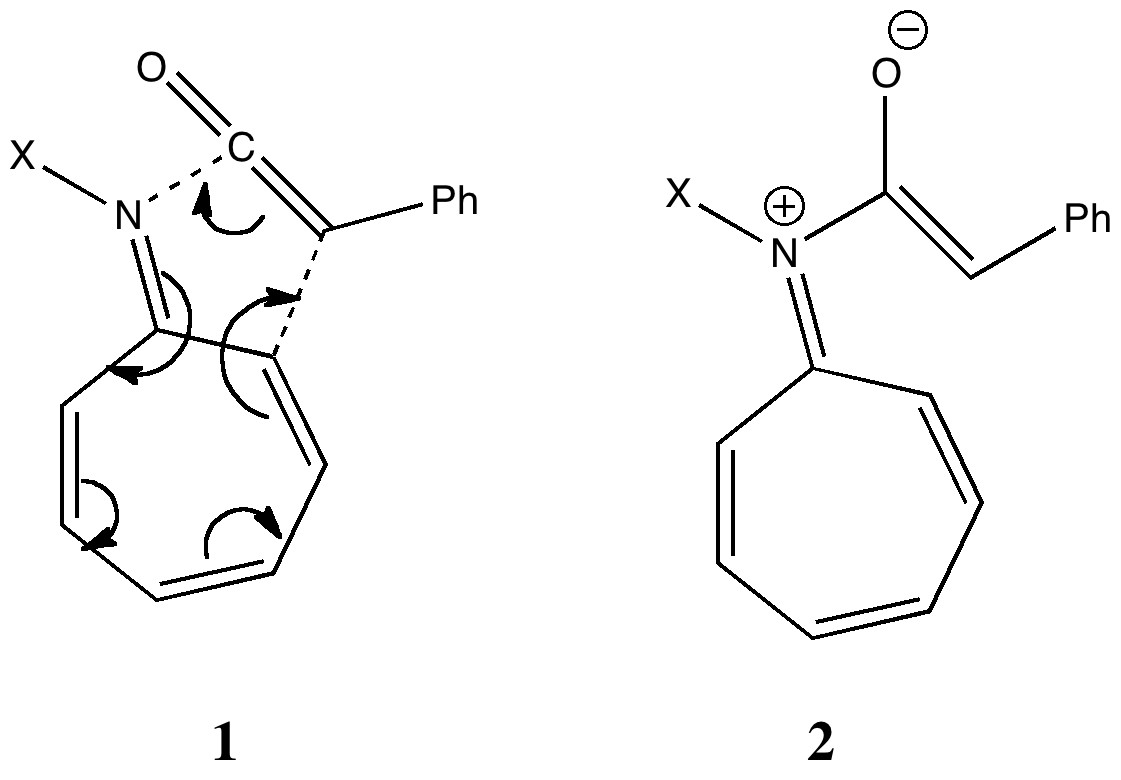
Steve Bachrach has blogged on the reaction shown below. If it were a pericyclic cycloaddition, both new bonds would form simultaneously, as shown with the indicated arrow pushing. Ten electrons would be involved, and in theory, the transition state would have 4n+2 aromaticity.
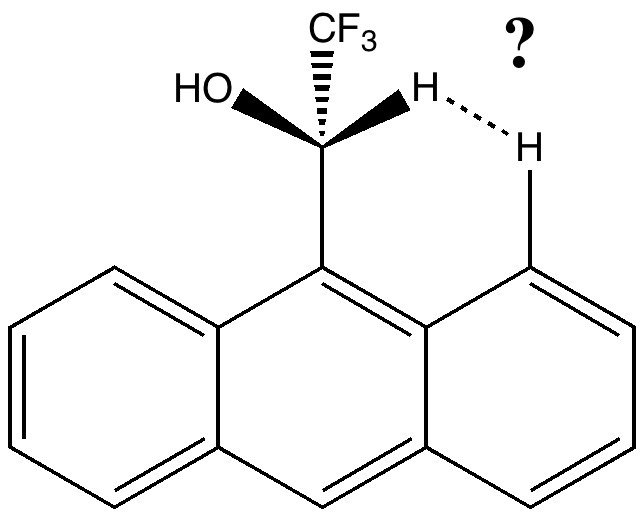
This molecule is not leaving me in peace. It and I first met in 1990 (DO: 10.1039/C39910000765), when we spotted the two unusual π-facial bonds formed when it forms a loose dimer.
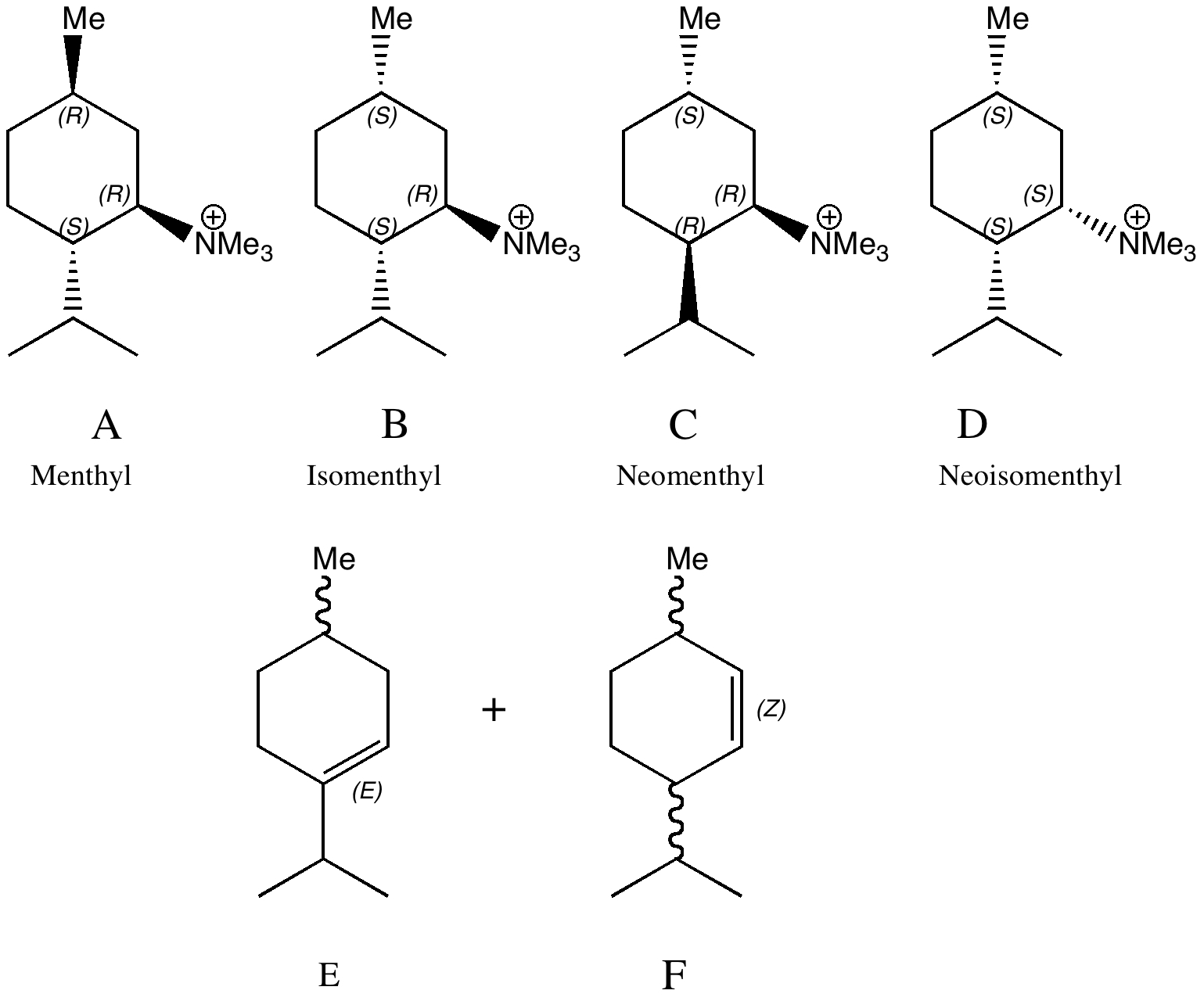
Conformational analysis comes from the classical renaissance of physical organic chemistry in the 1950s and 60s. The following problem is taken from E. D. Hughes and J. Wilby J. Chem. Soc. , 1960, 4094-4101, DOI: 10.1039/JR9600004094, the essence of which is that Hofmann elimination of a neomenthyl derivative (C below) was observed as anomalously faster than its menthyl analogue.
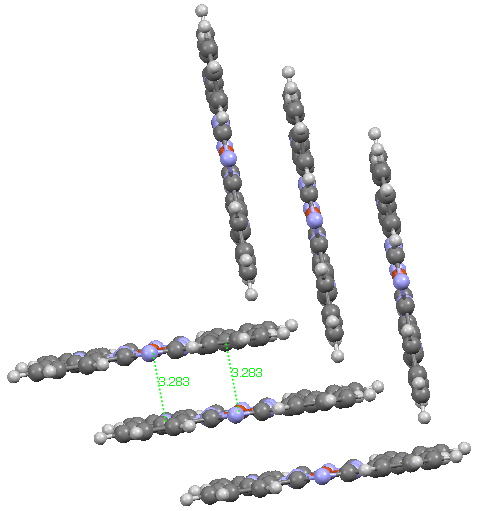
Andy Mclean posted a comment to my story of copper phthalocyanine (Monastral blue). The issue was its colour, and more specifically why this pigment has two peaks λ max 610 and 710nm making it blue. The first was accurately reproduced by calculation on the monomer, but the second was absent with such a model. Andy suggested this latter was due to stacking. Here, the calculated spectrum of a stacked dimer is explored.

Much of chemistry is about bonds, but sometimes it can also be about anti-bonds. It is also true that the simplest of molecules can have quite subtle properties. Thus most undergraduate courses in chemistry deal with how to describe the bonding in the diatomics of the first row of the periodic table.
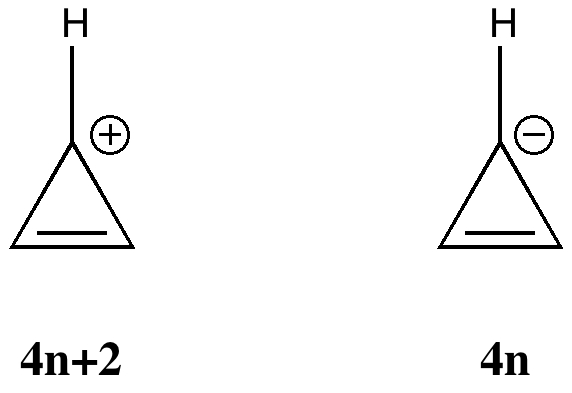
More inspiration from tutorials. In a lecture on organic aromaticity, the 4n+2/4n Hückel rule was introduced (in fact, neither rule appears to have actually been coined in this form by Hückel himself!). The simplest examples are respectively the cyclopropenyl cation and anion. The former has 2 π-electrons exhibiting cyclic delocalisation, and the 4n+2 (n=0) rule predicts aromaticity.
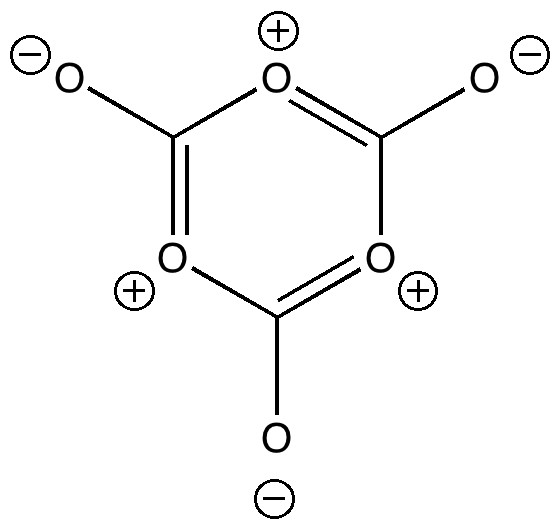
Many university chemistry departments, and mine is no exception, like to invite applicants to our courses to show them around. Part of the activities on the day is an “interview” in which the candidate is given a chance to shine. Over the years, I have evolved questions about chemistry which can form the basis of discussion, and I thought I would share one such question here.