Here is another example gleaned from that Woodward essay of 1967 ( Chem. Soc. Special Publications (Aromaticity), 1967 , 21 , 217-249), where all might not be what it seems. Woodward notes that the reaction between the (highly reactive) 1 does not occur.
Sometimes the originators of seminal theories in chemistry write a personal and anecdotal account of their work. Niels Bohr[cite]10.1007/BF01326955[/cite] was one such and four decades later Robert Woodward wrote “ The conservation of orbital symmetry ” (Chem. Soc. Special Publications (Aromaticity), 1967 , 21 , 217-249;
In the preceding post, I introduced Dewar’s π-complex theory for alkene-metal compounds, outlining the molecular orbital analysis he presented, in which the filled π-MO of the alkene donates into a Ag + empty metal orbital and back-donation occurs from a filled metal orbital into the alkene π* MO. Here I play a little “what if” game with this scenario to see what one can learn from doing so. Firstly, I will use
The period 1951–1954 was a golden one for structural chemistry; proteins, DNA, Ferrocene (1952) and the one I discuss here, a bonding model for Zeise’s salt ( 3 ). In “A review of π Complex Theory”, Bull. Soc. Chim. Fr. , 1951 , 1 8 , C79 (it is not online) M. J. S. Dewar sets out his theory of the role of π-complexes in (mostly) organic chemistry.
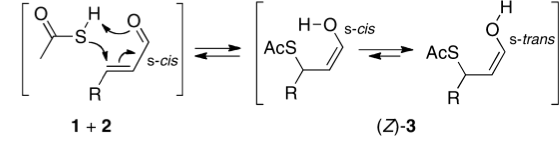
Lukas, who occasionally comments on this blog, sent me the following challenge. In a recent article[cite]10.1021/jo3021709[/cite] he had proposed that the stereochemical outcome ( Z ) of reaction between a butenal and thioacetic acid as shown below arose by an unusual concerted cycloaddtion involving an S-H bond.
The previous post described how the acid catalysed ring opening of propene epoxide by an alcohol (methanol) is preceded by pre-protonation of the epoxide oxygen to form a “hidden intermediate” on the concerted intrinsic reaction pathway to ring opening.
In a previous post on the topic, I remarked how the regiospecific ethanolysis of propene epoxide[cite]10.1021/ja01208a047[/cite] could be quickly and simply rationalised by inspecting the localized NBO orbital calculated for either the neutral or the protonated epoxide. This is an application of Hammond’s postulate[[cite]10.1021/ja01607a027[/cite] in extrapolating the properties of a reactant to its reaction transition state.
A few posts back, I explored the “benzidine rearrangement” of diphenyl hydrazine. This reaction requires diprotonation to proceed readily, but we then discovered that replacing one NH by an O as in N,O-diphenyl hydroxylamine required only monoprotonation to undergo an equivalent facile rearrangement. So replacing both NHs by O to form diphenyl peroxide (Ph-O-O-Ph) completes this homologous series.
I recently got an email from a student asking about the best way of rationalising epoxide ring opening using some form of molecule orbitals.
This is another in the occasional series of “what a neat molecule”. In this case, more of a “what a neat idea”. The s-triazine below, when coordinated to eg ZnI 2 , forms what is called a metal-organic-framework, or MOF.
