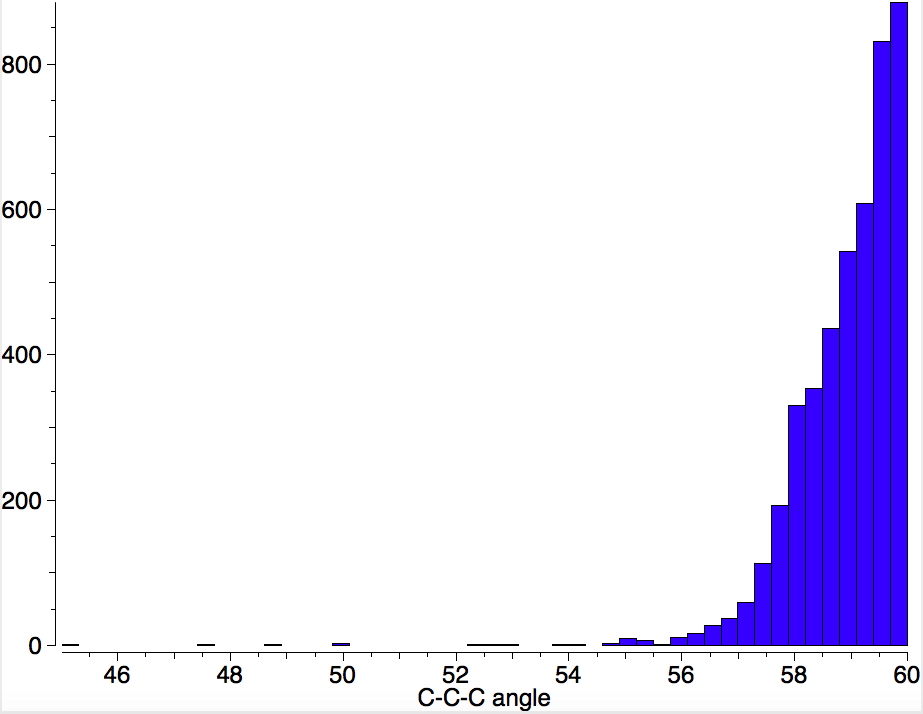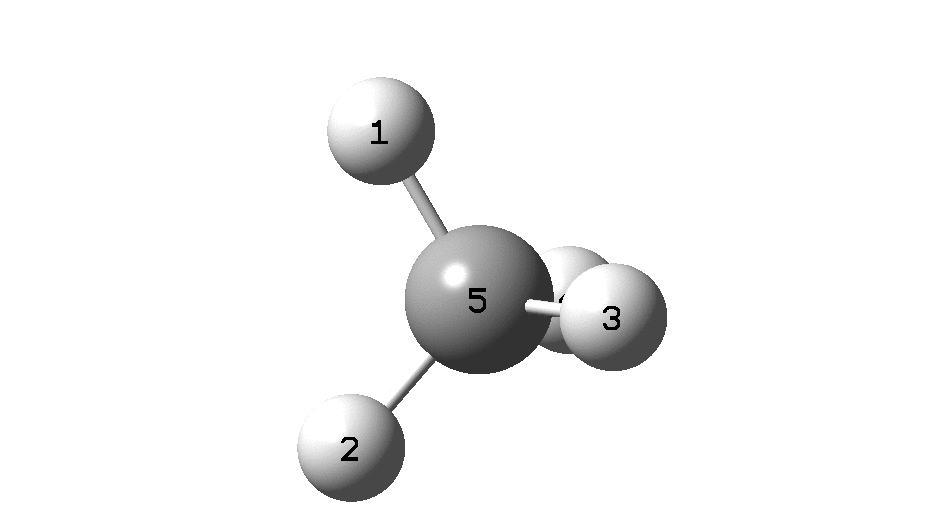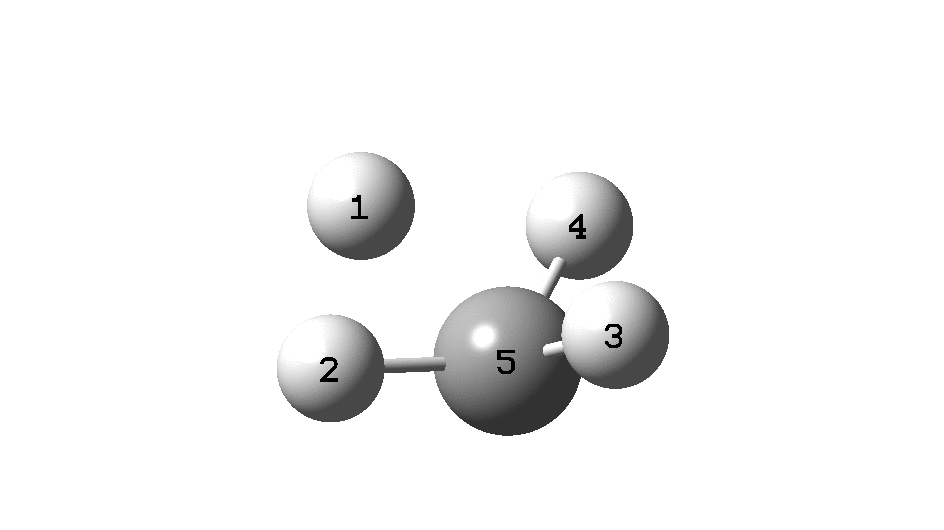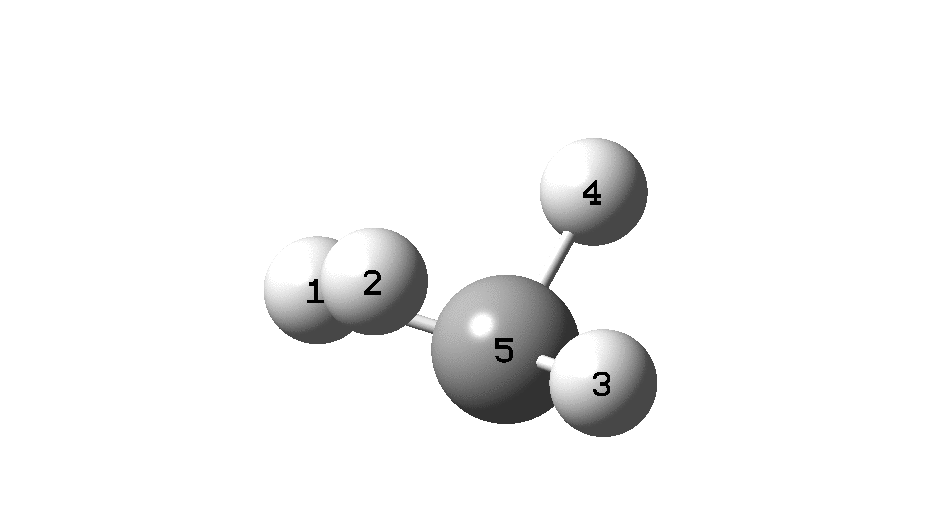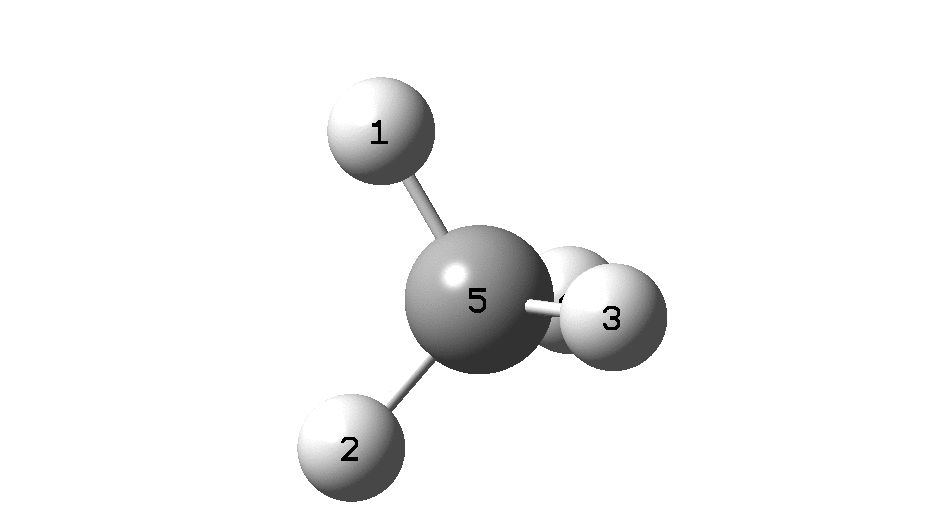
This is a spin-off from the table I constructed here for further chemical examples of the classical/non-classical norbornyl cation conundrum. One possible entry would include the transition state for inversion of methane via a square planar geometry as compared with e.g. NiH 4 for which the square planar motif is its minimum.