The Bürgi–Dunitz angle describes the trajectory of an approaching nucleophile towards the carbon atom of a carbonyl group. A colleague recently came to my office to ask about the inverse, that is what angle would an electrophile approach (an amide)? Thus it might approach either syn or anti with respect to the nitrogen, which is a feature not found with nucleophilic attack.
I was reminded of this article by Michelle Francl[cite]10.1038/nchem.1733[/cite], where she poses the question “What anchor values would most benefit students as they seek to hone their chemical intuition?” She gives as common examples: room temperature is 298.17K (actually 300K, but perhaps her climate is warmer than that of the UK!), the length of a carbon-carbon single bond, the atomic masses of the more common elements.
A word of explanation about this test page for experimenting with JSmol. Many moons ago I posted about how to include a generated 3D molecular model in a blog post, and have used that method on many posts here ever since. It relied on Java as the underlying software (first introduced in 1996), or almost 20 years ago.
Following the discussion here of Kekulé’s suggestion of what we now call a vibrational mode (and which in fact now bears his name), I thought I might apply the concept to a recent molecule known as [2.2]paracyclophane.
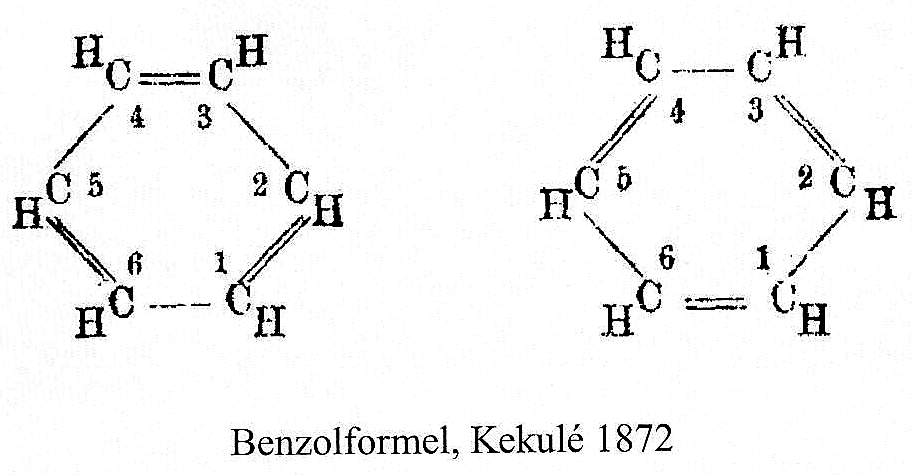
In the preceding post, a nice discussion broke out about Kekulé’s 1872 model for benzene.[cite]10.1002/jlac.18721620110[/cite] This model has become known as the oscillation hypothesis between two extreme forms of benzene (below). The discussion centered around the semantics of the term oscillation compared to vibration (a synonym or not?) and the timescale implied by each word.
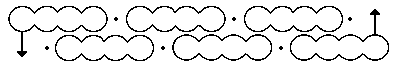
Continuing my european visits, here are two photos from Bonn. First, a word about how the representation of benzene evolved, attributed to Kekulé. The sausage formula Above is his first effort, made in 1865. The bent bond formula This one above is better, offered in 1866.
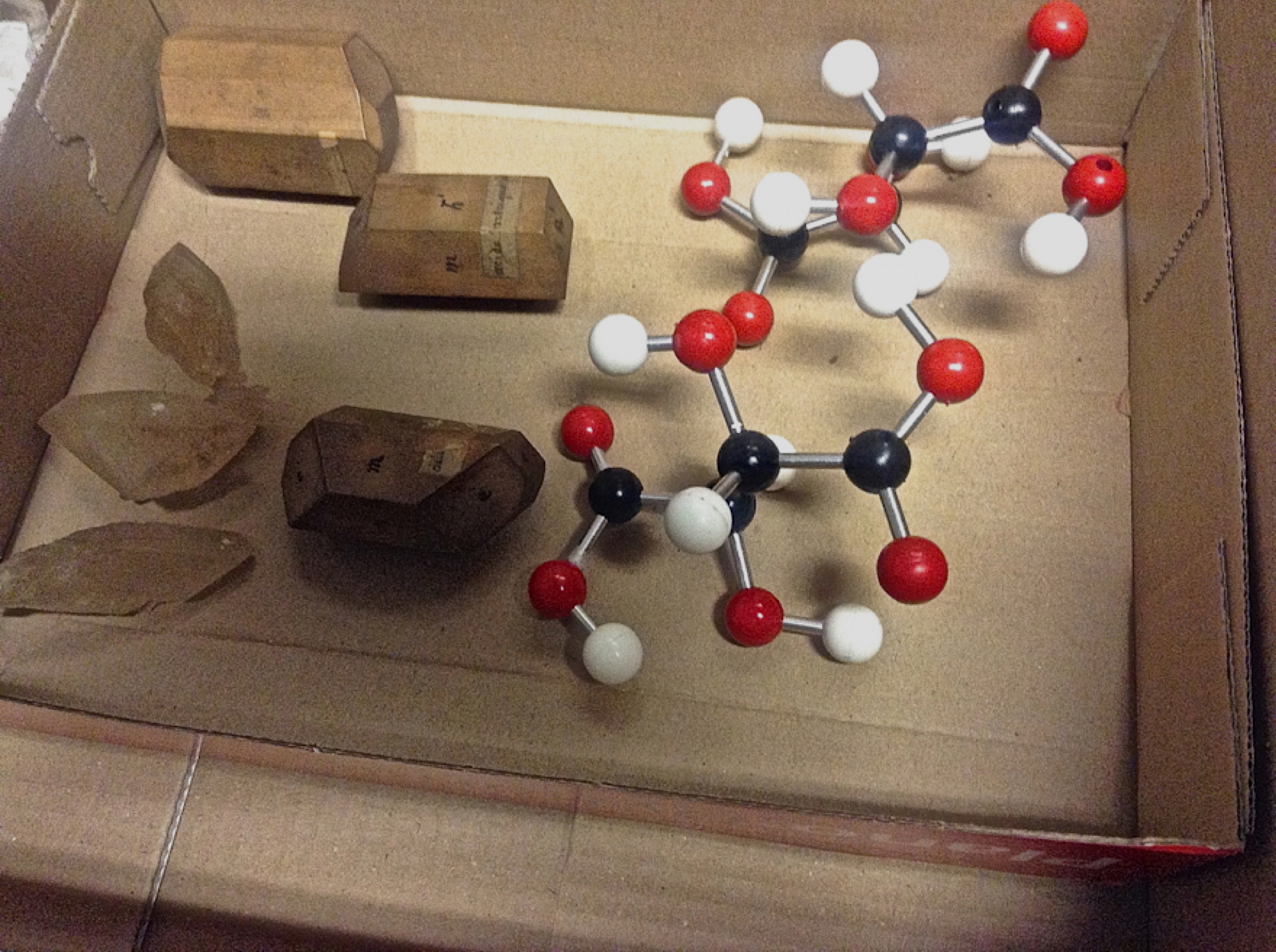
Not a computer in sight! I refer to a chemistry lab from the 1800s I was recently taken to, where famous french chemists such as Joseph Gay-Lussac, Michel Chevreul and Edmond Fremy were professors. Although not used for chemistry any more, it is an incredible treasure trove of objects. Here are photos of some.
I remember a time when tracking down a particular property of a specified molecule was an all day effort, spent in the central library (or further afield). Then came the likes of STN Online (~1980) and later Beilstein. But only if your institution had a subscription.
My name is displayed pretty prominently on this blog, but it is not always easy to find out who the real person is behind many a blog. In science, I am troubled by such anonymity.

This is rather cranking the handle, but taking my previous post and altering the search definition of the crystal structure database from 4- to 5-coordinate metals, one gets the following. Fe … Co … Ni … Cu … Trigonal bipyramidal coordination has angles of 90, 120 and 180°. Square pyramidal has no 120° angles, and the 180° angles might be somewhat reduced.
