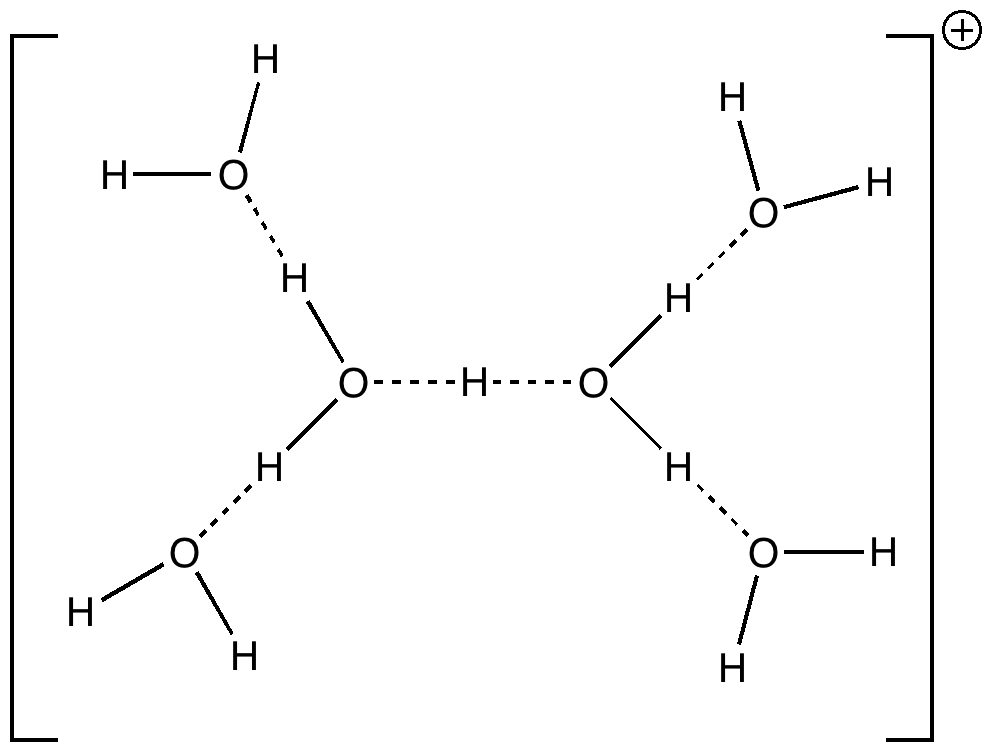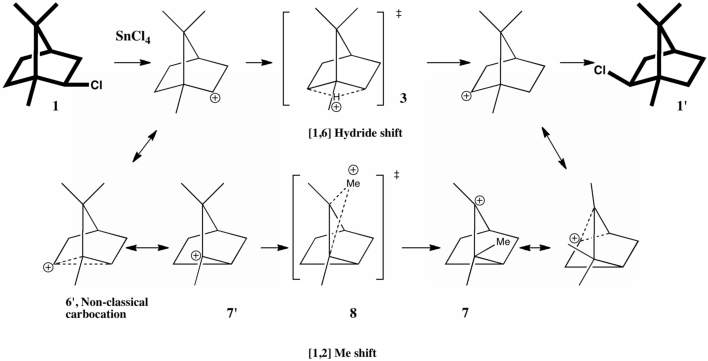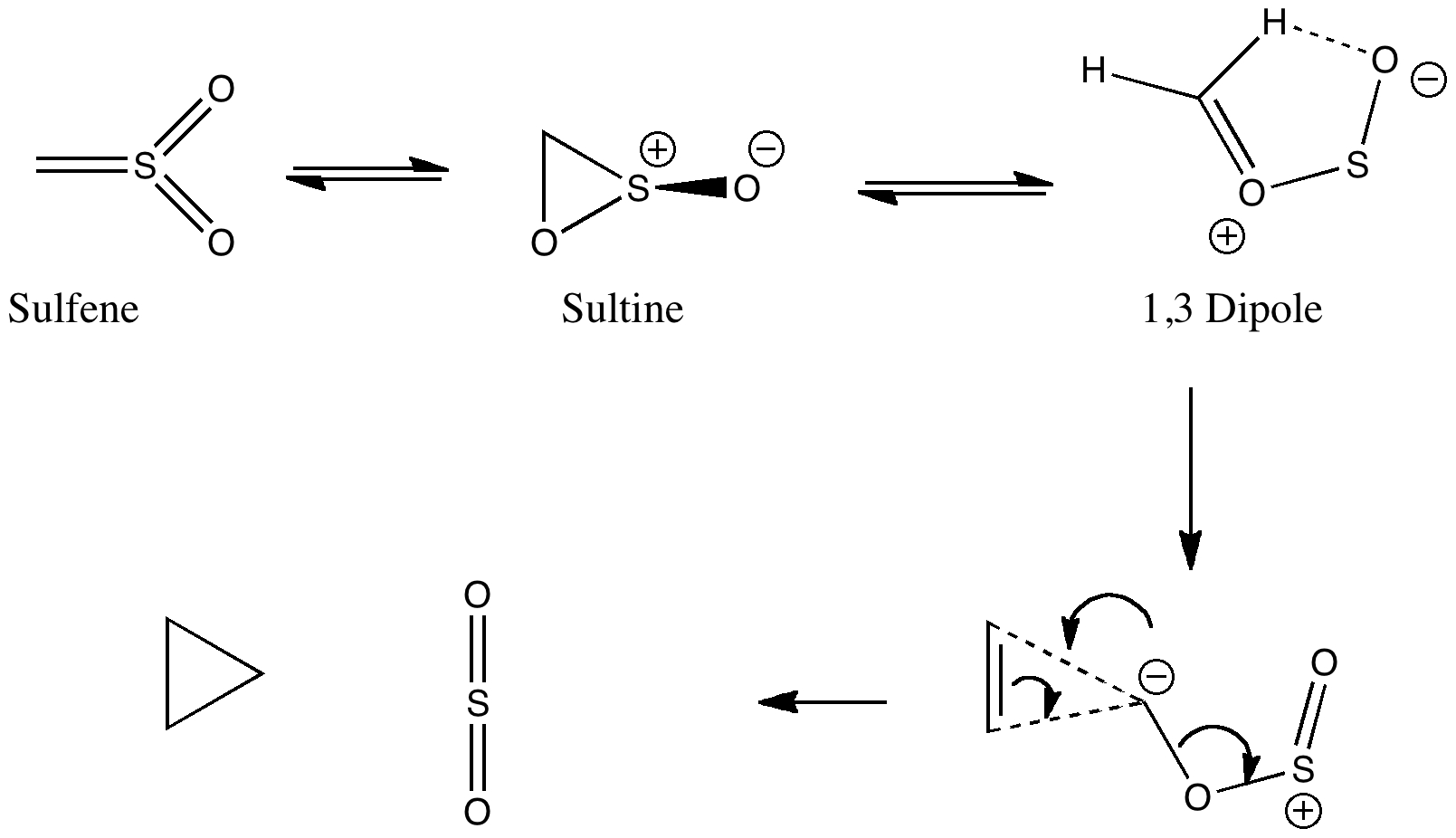
One future vision for chemistry over the next 20 years or so is the concept of having machines into which one dials a molecule , and as if by magic, the required specimen is ejected some time later. This is in some ways an extrapolation of the existing peptide and nucleotide synthesizer technologies and sciences.