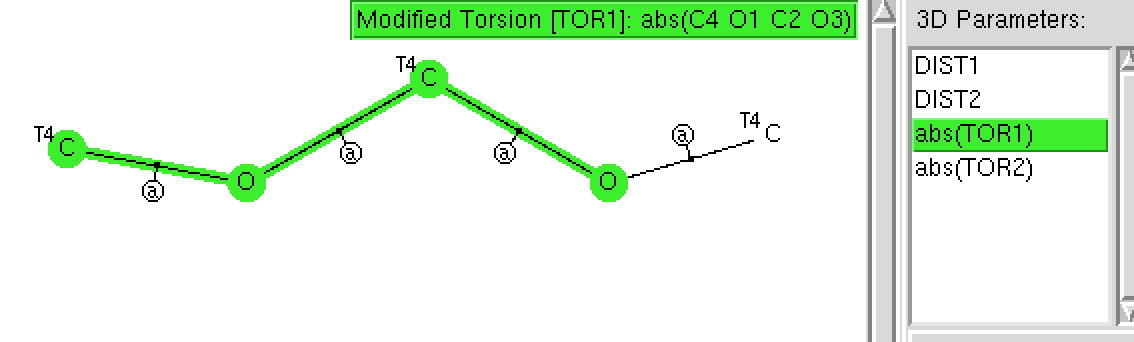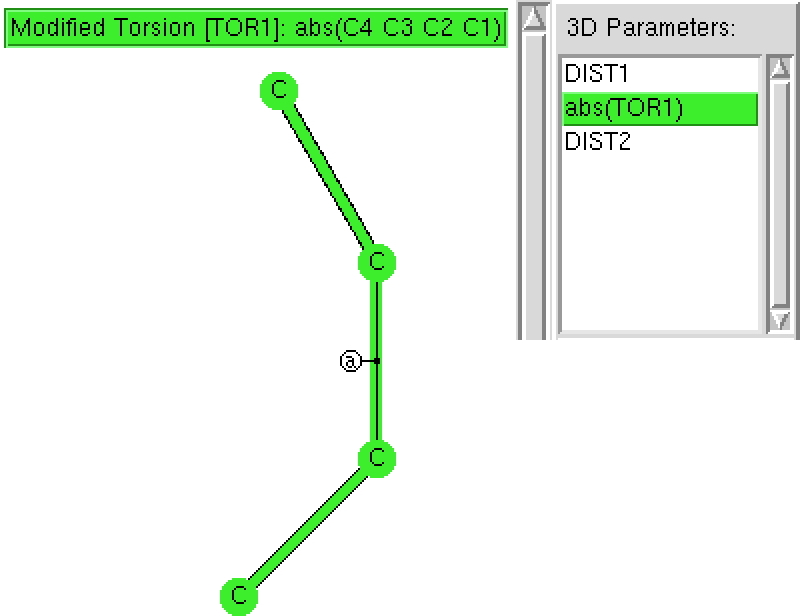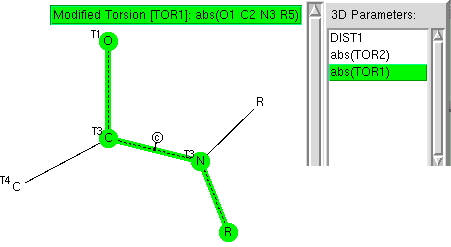
The π-resonance in amides famously helped Pauling to his proposal of a helical structure for proteins. Here I explore some geometric properties of amides related to the C-N bond and the torsions about it. The key aspect of amides is that a lone pair of electrons on the nitrogen can conjugate with the C=O carbonyl only if the lone pair orbital is parallel to the C-O π-system.