Derek Lowe has a recent post entitled " Another Funny-Looking Structure Comes Through ". He cites a recent medchem article[cite]10.1021/acsmedchemlett.5b00398[/cite] in which the following acetal sub-structure appears in a promising drug candidate (blue component below). His point is that orally taken drugs have to survive acid (green below) encountered in the stomach, and acetals are famously sensitive to hydrolysis (red below). But if

Steve Bachrach on his own blog has commented on a recent article[cite]10.1002/anie.201505934[/cite] discussing the structure of the trimer of fluoroethanol. Rather than the expected triangular form with three OH—O hydrogen bonds, the lowest energy form only had two such bonds, but it matched the microwave data much better. Here I explore this a bit more.

I have previously shown the grave of William Perkin, a great british organic chemist. On a recent visit to Paris, I went to see the crypt in the Panthéon, the great french secular necropolis. What a contrast to Perkin! The Curies have a crypt all to themselves (VII), and other great french scientists such as Bertholet and Langevin as well as mathematicians such as Lagrange who are also interred in other crypts.

In Jingdezhen an Imperial Kiln was built in 1369 to produce porcelain that was “white as jade, thin as paper, bright as a mirror and tuneful as a bell”. It’s the colours of the glazes that caught my eye, achieved by a combination of oxidative and reductive firing in the kiln, coupled with exquisite control of the temperature.

This comes to you from China, and the city of Suzhou. To set the scene, cities in China have a lot of motorbikes. Electric ones. With their own speed units, a % of Panda speed. Msny msny people ride bikes such as these; some even manage three passengers, or several boxes of shopping. And the streets will have dedicated lanes for them, although you do need eyes in the back of your head to spot their silent (often 15 kph) approach.
A fascinating re-examination has appeared[cite]10.1002/anie.201505482[/cite] of a reaction first published[cite]10.1002/ange.19600721210[/cite] in 1960 by Wittig and then[cite]10.1002/jlac.19646790106[/cite] repudiated by him in 1964 since it could not be replicated by a later student.
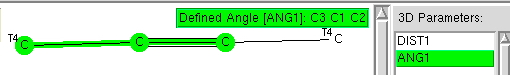
Previously, I explored deviation from ideal tetrahedral arrangements of four carbon ligands around a central (sp 3 ) carbon using crystal structures. Now it is the turn of digonal (sp 1 ) and trigonal (sp 2 ) carbons. Firstly, the digonal C≡C case. Attached to each carbon of the C≡C unit are two saturated carbon ligands; this to prevent conjugation from influencing our result.
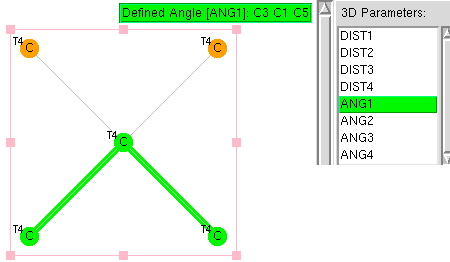
An article entitled " Four Decades of the Chemistry of Planar Hypercoordinate Compounds "[cite]10.1002/anie.201410407[/cite] was recently reviewed by Steve Bacharach on his blog, where you can also see comments. Given the recent crystallographic themes here, I thought I might try a search of the CSD (Cambridge structure database) to see whether anything interesting might emerge for tetracoordinate carbon.
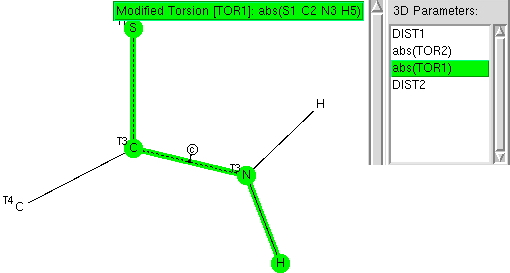
The previous post explored the structural features of amides. Here I compare the analysis with that for the closely related thioamides. Here is the torsional analysis around the C-N bond. The “diff” (difference) is that almost all the hits are concentrated into angles of 0° or 180°; the twist about the C-N bond from co-planarity is much less if S is present.
