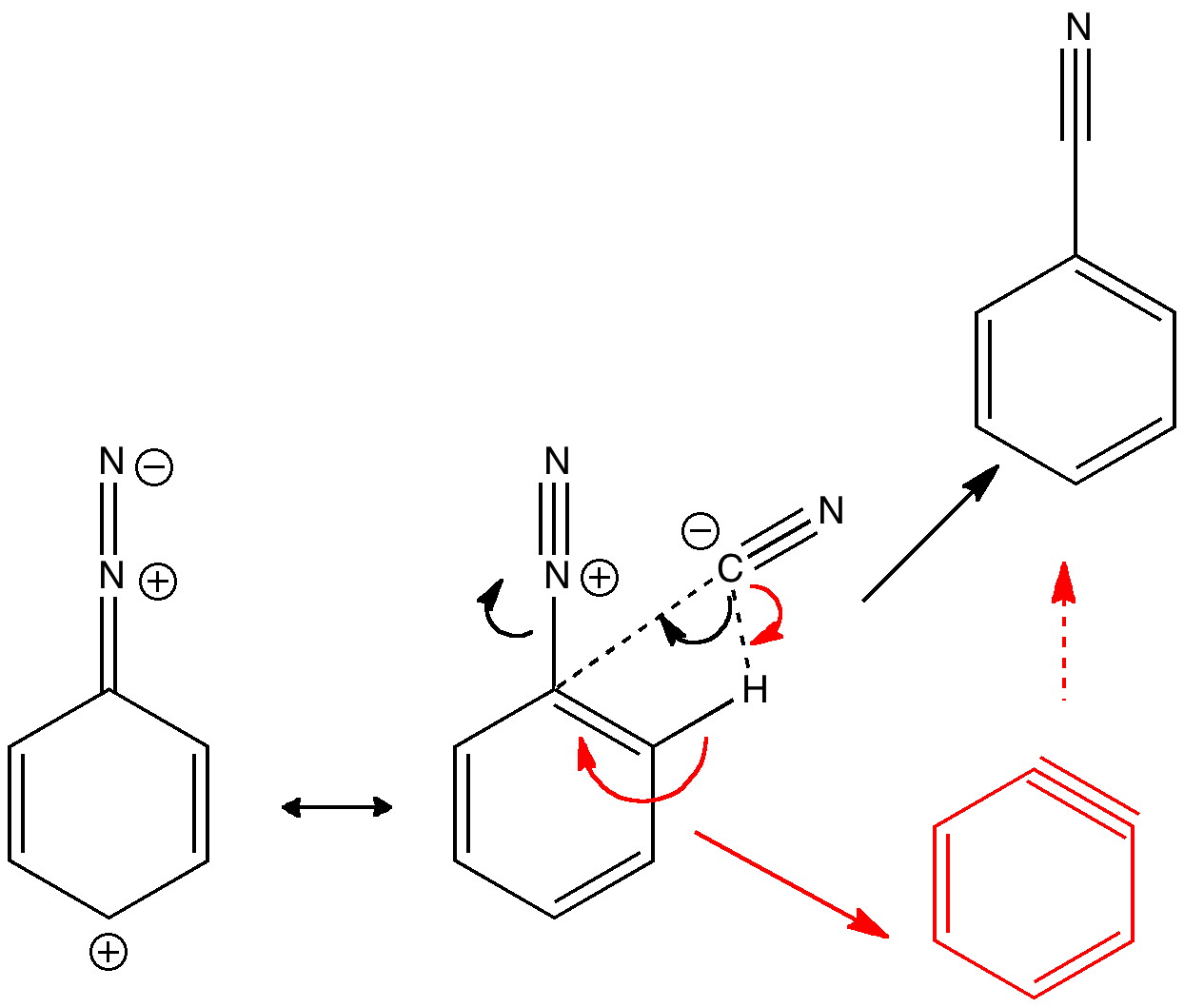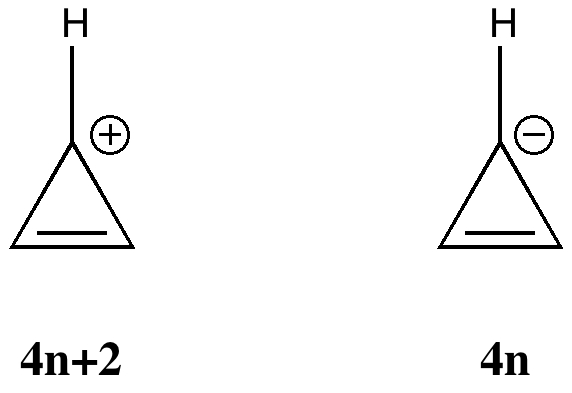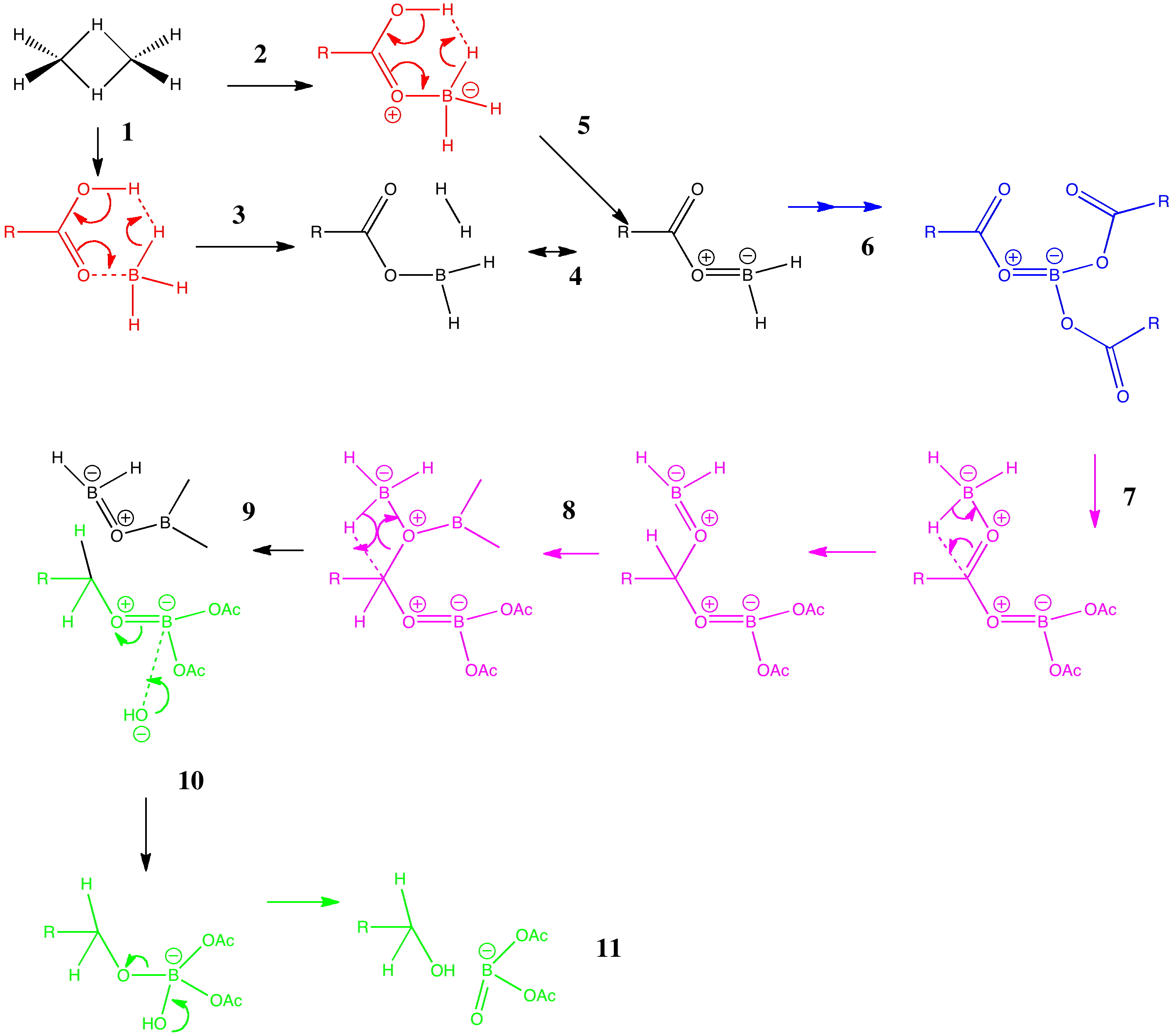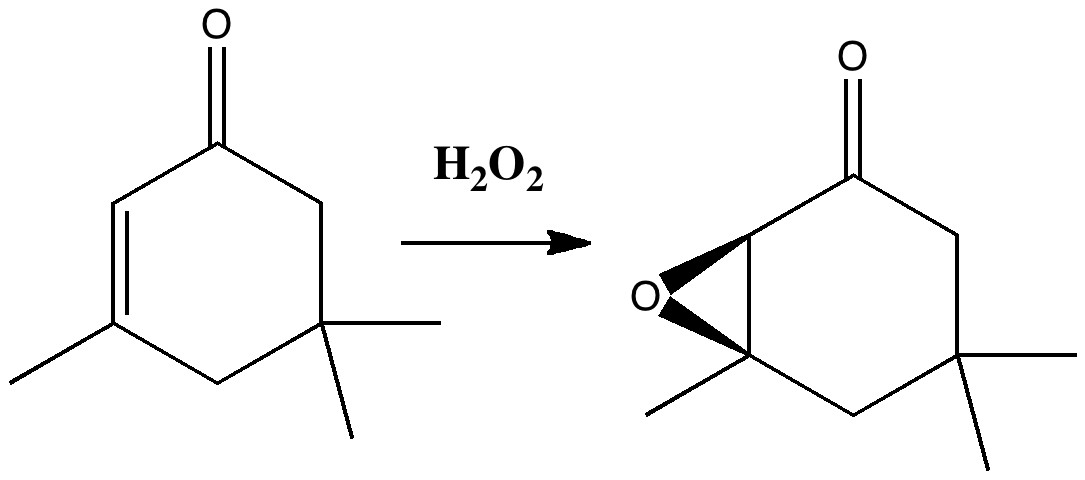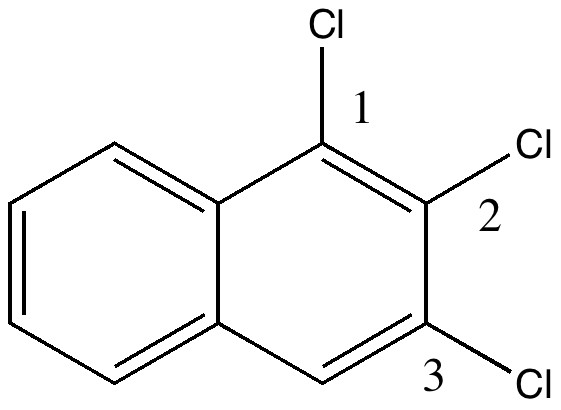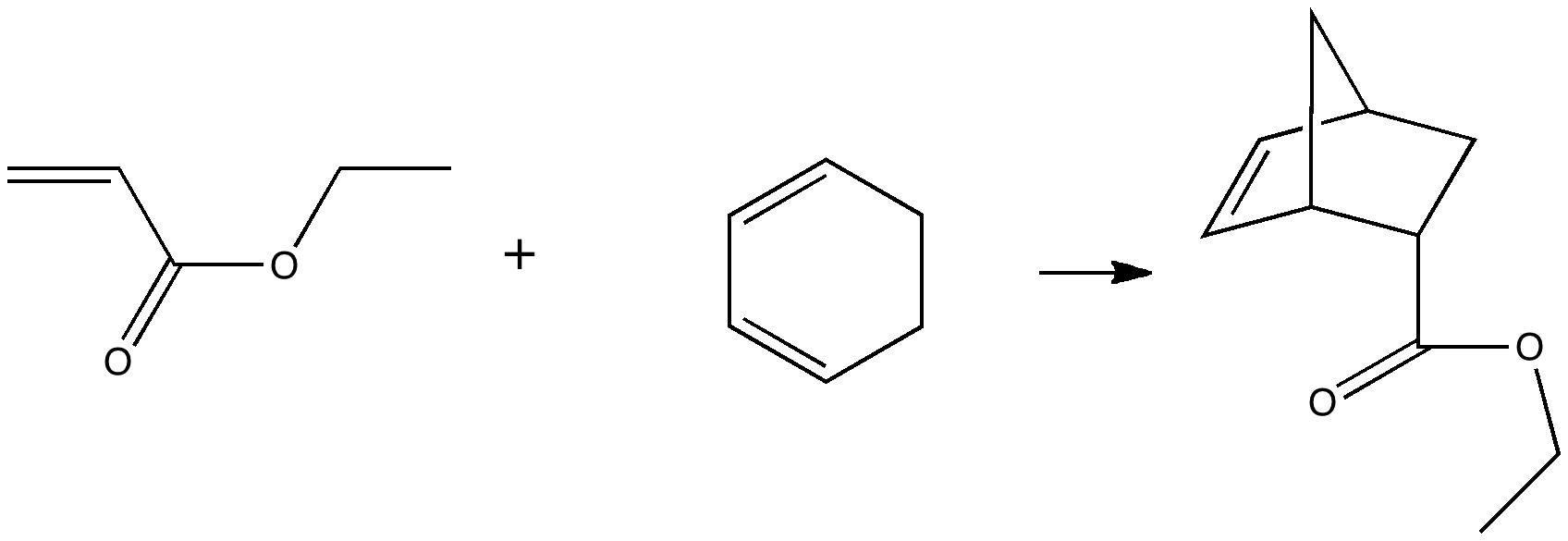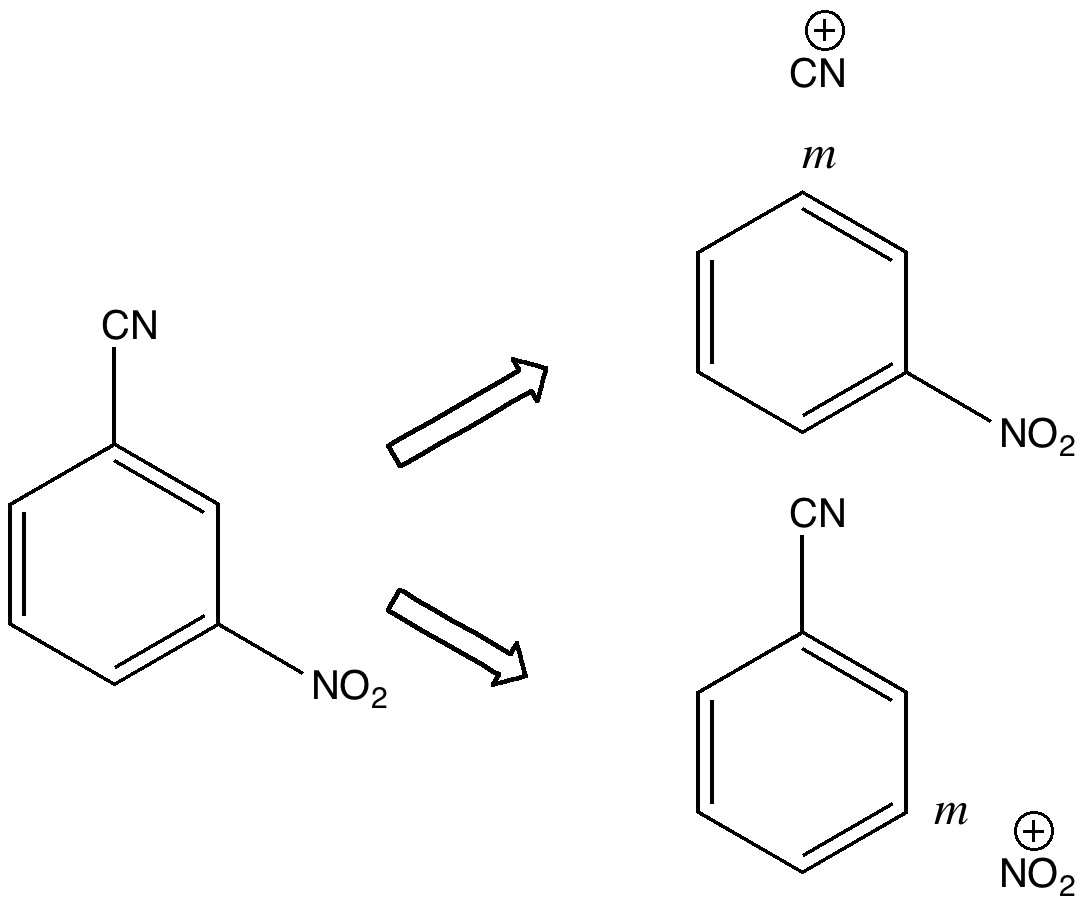
Do you fancy a story going from simplicity to complexity, if not absurdity, in three easy steps? Read on! The following problem appears in one of our (past) examination questions in introductory organic chemistry. From relatively mundane beginnings, one can rapidly find oneself in very unexpected territory. How would one make 3-nitrobenzonitrile?