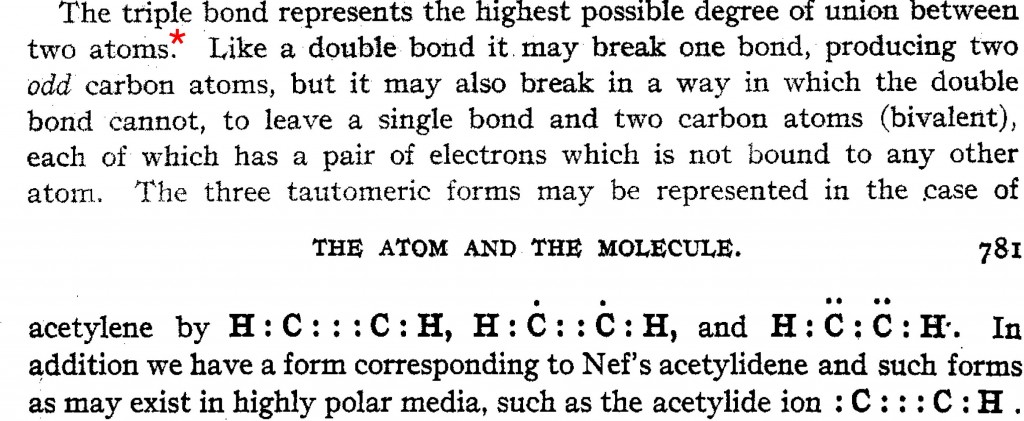
The upcoming ACS national meeting in San Diego has a CHED (chemical education division) session entitled Implementing Discovery-Based Research Experiences in Undergraduate Chemistry Courses. I had previously explored what I called extreme gauche effects in the molecule F-S-S-F. Here I take this a bit further to see what else can be discovered about molecules containing bonds between group 16 elements (QA= O, S, Se, Te).