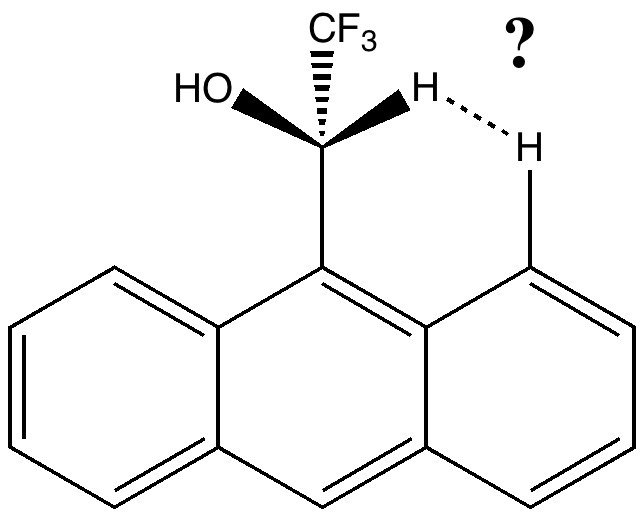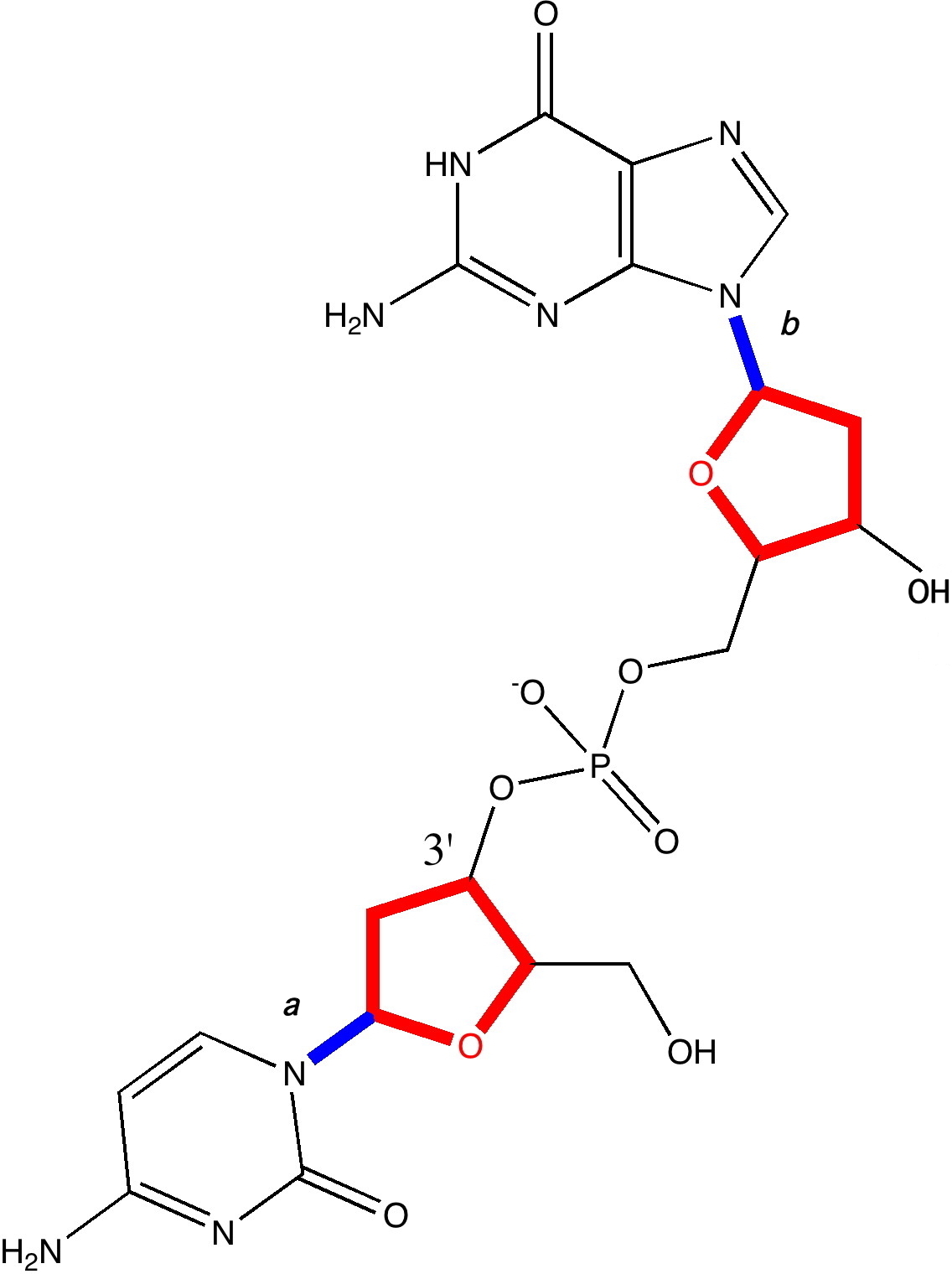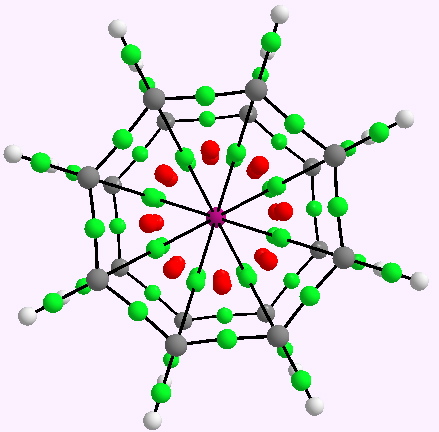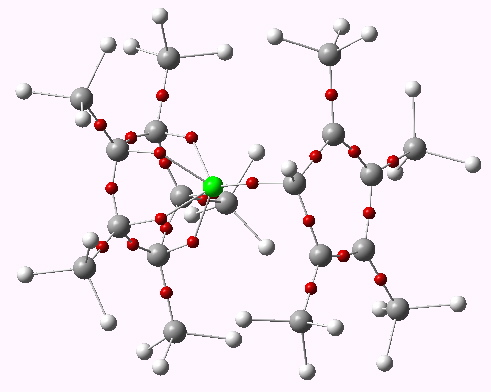
Observation of the slow racemization of isobornyl chloride in a polar solvent in 1923-24 by Meerwein led to the recognition that mechanistic interpretation is the key to understanding chemical reactivity. The hypothesis of ion pairs in which a chloride anion is partnered by a carbocation long ago entered the standard textbooks (see DOI 10.1021/ed800058c and 10.1021/jo100920e for background reading).