My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down. The barrier to their interconversion in this case is generated by a rotation about the two single bonds connecting the carbonyl group to the rest of the molecule.
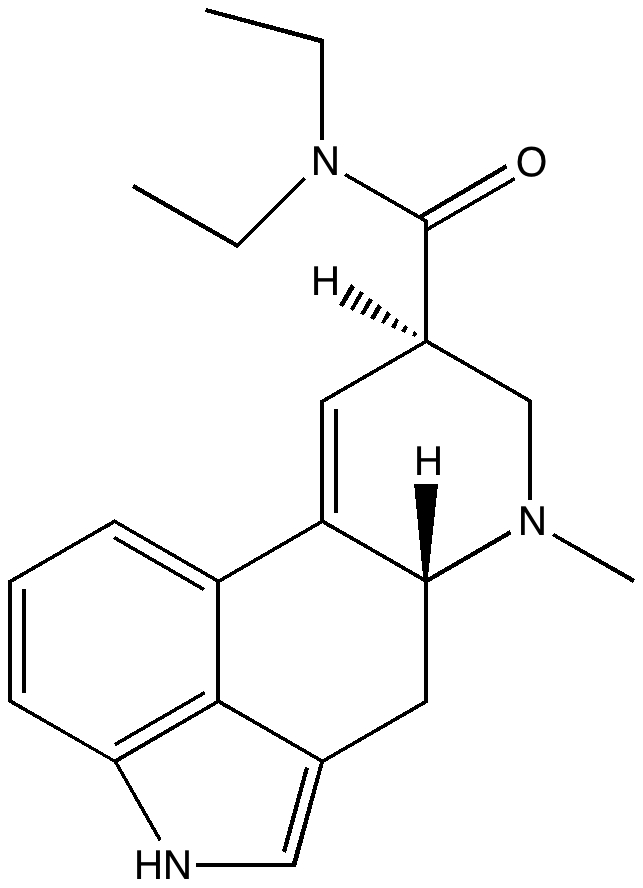
Moore’s law describes a long-term trend in the evolution of computing hardware, and it is often interpreted in terms of processing speed. Here I chart this rise in terms of the size of computable molecules. By computable I mean specifically how long it takes to predict the geometry of a given molecule using a quantum mechanical procedure. LSD, the 1975 benchmark for computable molecules.
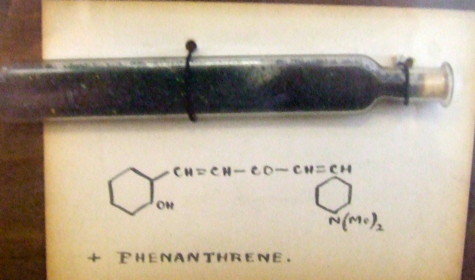
Organic chemists have been making (more or less pure) molecules for the best part of 180 years. Occasionally, these ancient samples are unearthed in cupboards, and then the hunt for their origin starts. I have previously described tracking down the structure of a 120 year-old sample of a naphthalene derivative. But I visited a colleague's office today, and recollected having seen a well-made wooden display cabinet there on a previous visit.
I asked a while back whether blogs could be considered a serious form of scholarly scientific communication (and so has Peter Murray-Rust more recently). A case for doing so might be my post of about a year ago, addressing why borane reduces a carboxylic acid, but not its ester, where I suggested a possible mechanism.
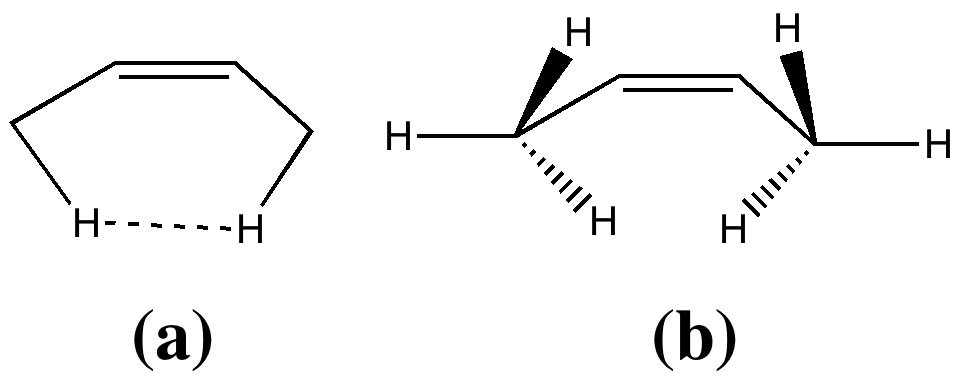
In two previous posts, I have looked at why cis -butene adopts conformation (a) rather than (b). I suggested it boiled down to electronic interactions between the methyl groups and the central alkene resulting in the formation of a H…H “ topological ” bond, rather than attraction between the H…H region to form a weak chemical “ bond “. Here I take a look at what happens when that central C=C bond is gradually removed.
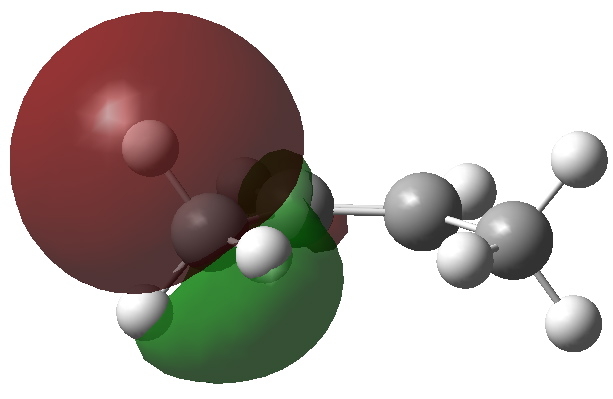
I wrote earlier about the strangely close contact between two hydrogen atoms in cis -butene. The topology of the electron density showed characteristics of a bond, but is it a consensual union?

The properties of electrons are studied by both chemists and physicists. At the boundaries of these two disciplines, sometimes interesting differences in interpretation emerge. One of the most controversial is that due to Bader (for a recent review, see DOI: 10.1021/jp102748b) a physicist who brought the mathematical rigor of electronic topology to bear upon molecules.
To (mis)quote Oscar Wilde again, ““To lose one methyl group may be regarded as a misfortune; to lose both looks like carelessness.” Here, I refer to the (past) tendency of molecular modellers to simplify molecular structures. Thus in 1977, quantum molecular modelling, even at the semi-empirical level, was beset by lost groups. One of my early efforts (DOI: 10.1021/ja00465a005) was selected for study because it had nothing left to lose;
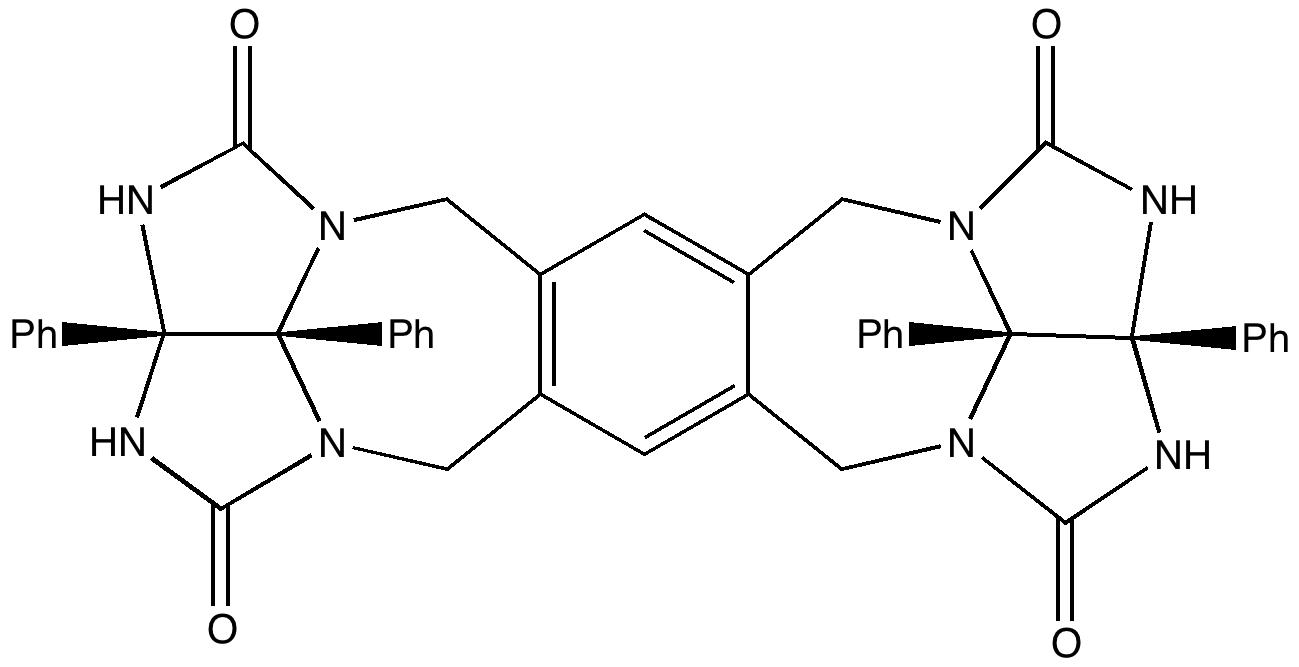
A Matryoshka doll is better known as a Russian nesting doll. They can have up to eight layers. Molecules can only emulate two layers, although see here for a good candidate for making a three-layered example (the inside layer is C 60 , which itself might encapsulate a small molecule. See also DOI: 10.1021/ja991747w). These molecular dolls can be created out of quite simple molecules.
Organic chemistry has some no-go areas, where few molecules dare venture. One of them is described by a concept known as anti-aromaticity. Whereas aromatic molecules are favoured species, their anti-equivalent is avoided. I previously illustrated this (Hückel rule) with cyclopropenium anion.
