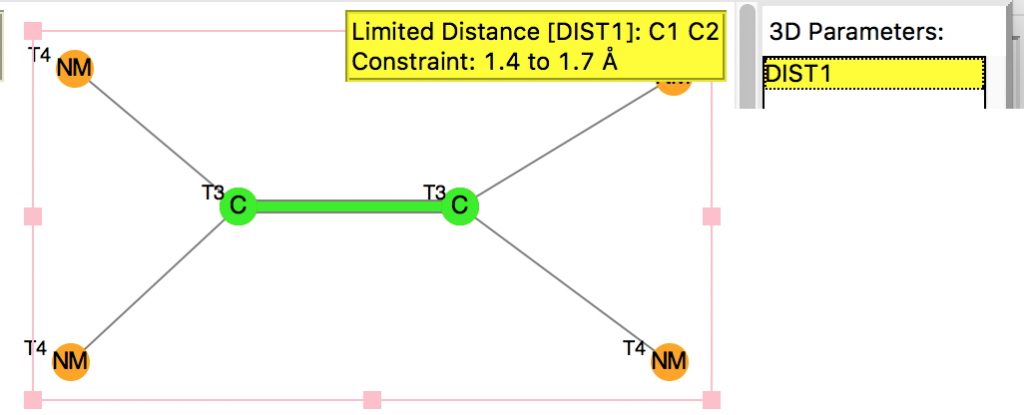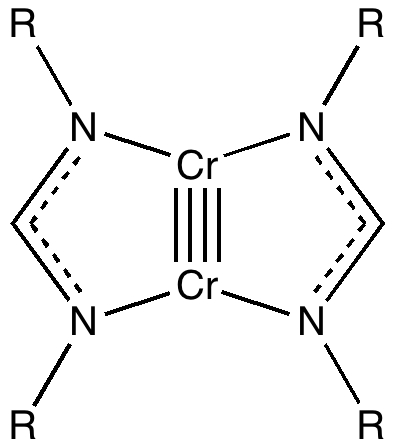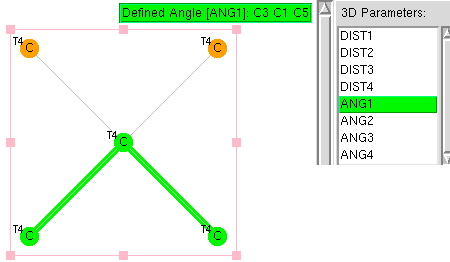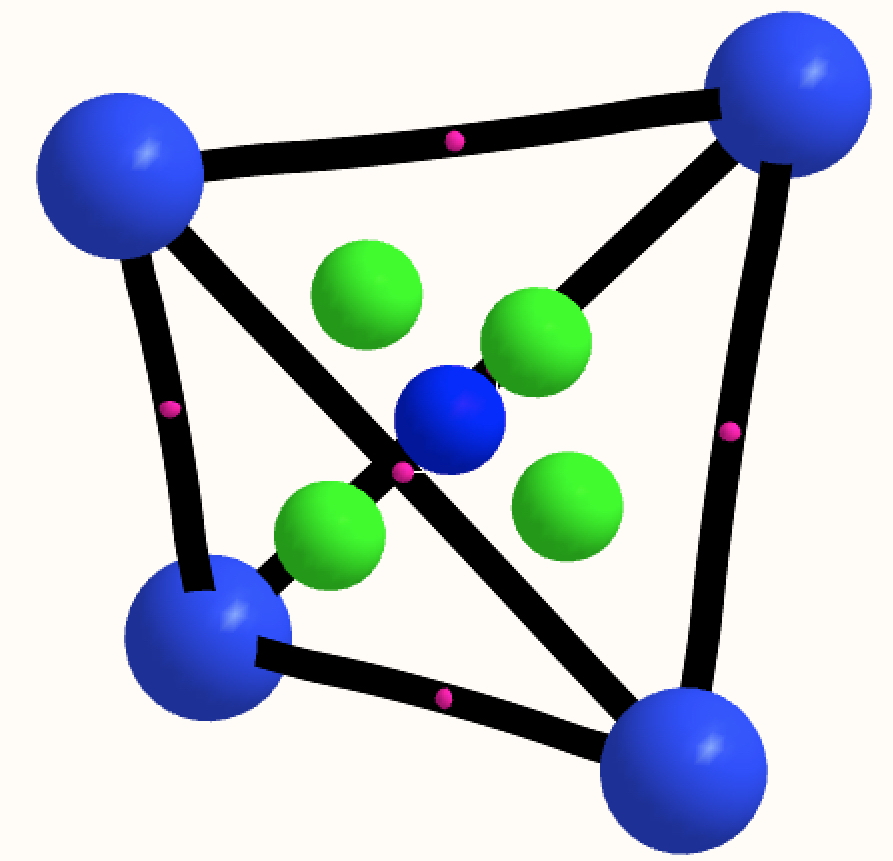
The thread thus far. The post about Na 2 He introduced the electride anionic counter-ion to Na + as corresponding topologically to a rare feature known as a non-nuclear attractor. This prompted speculation about other systems with such a feature, and the focus shifted to a tetrahedral arrangement of four hydrogen atoms as a dication, sharing a total of two valence electrons. The story now continues here.