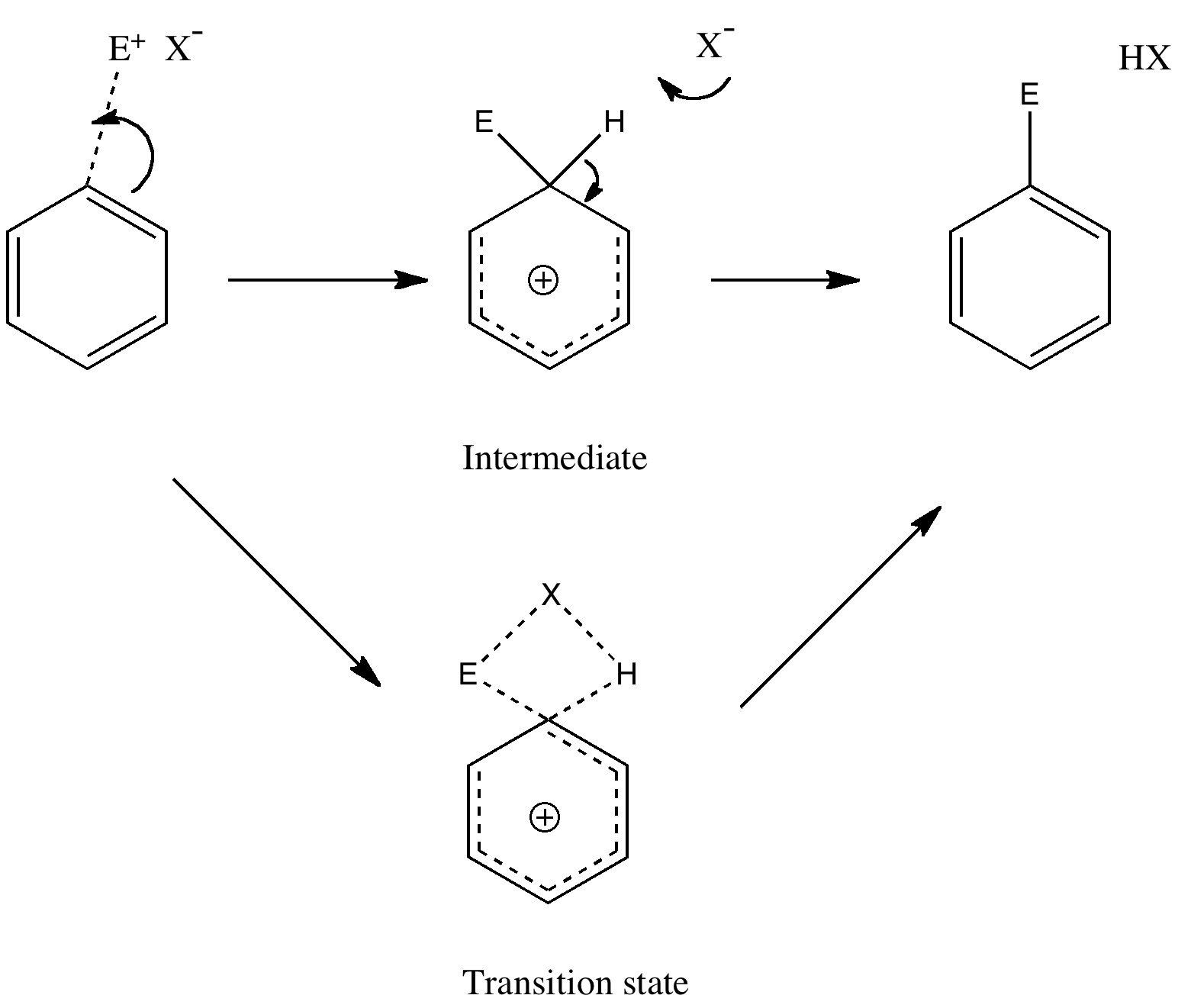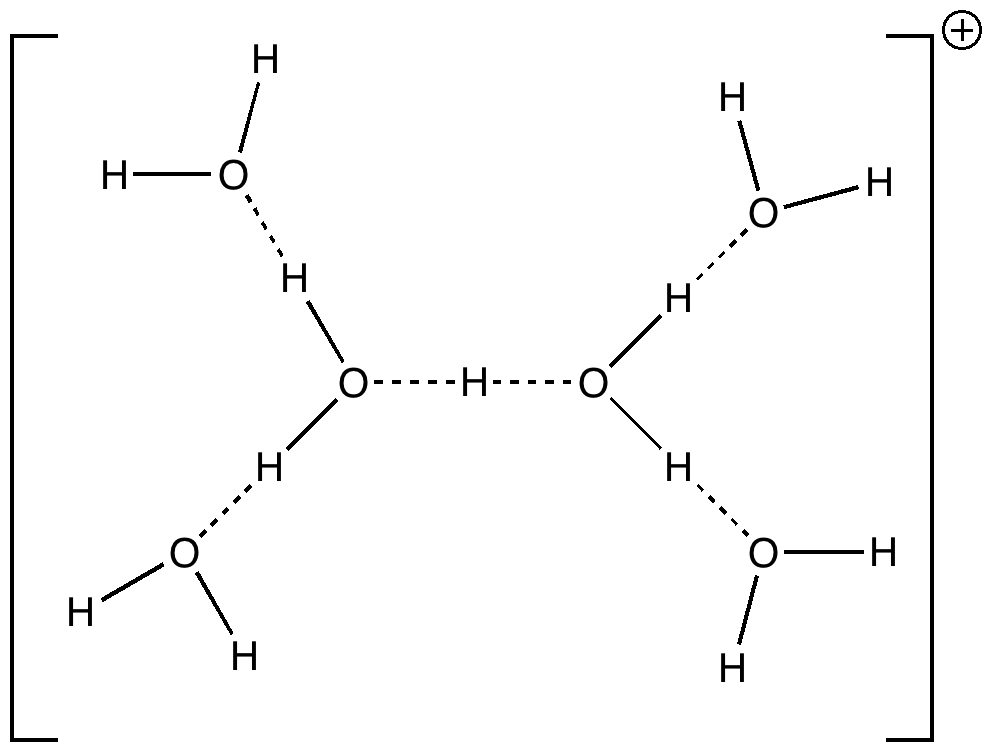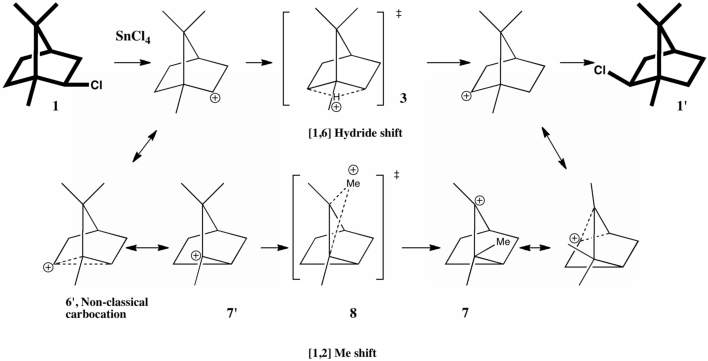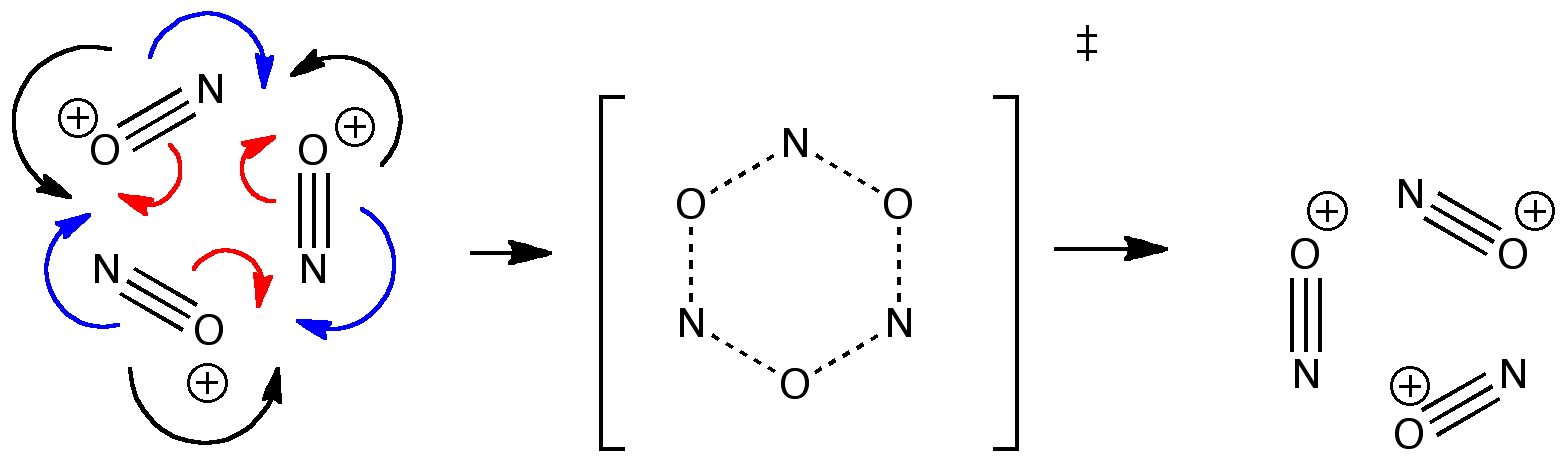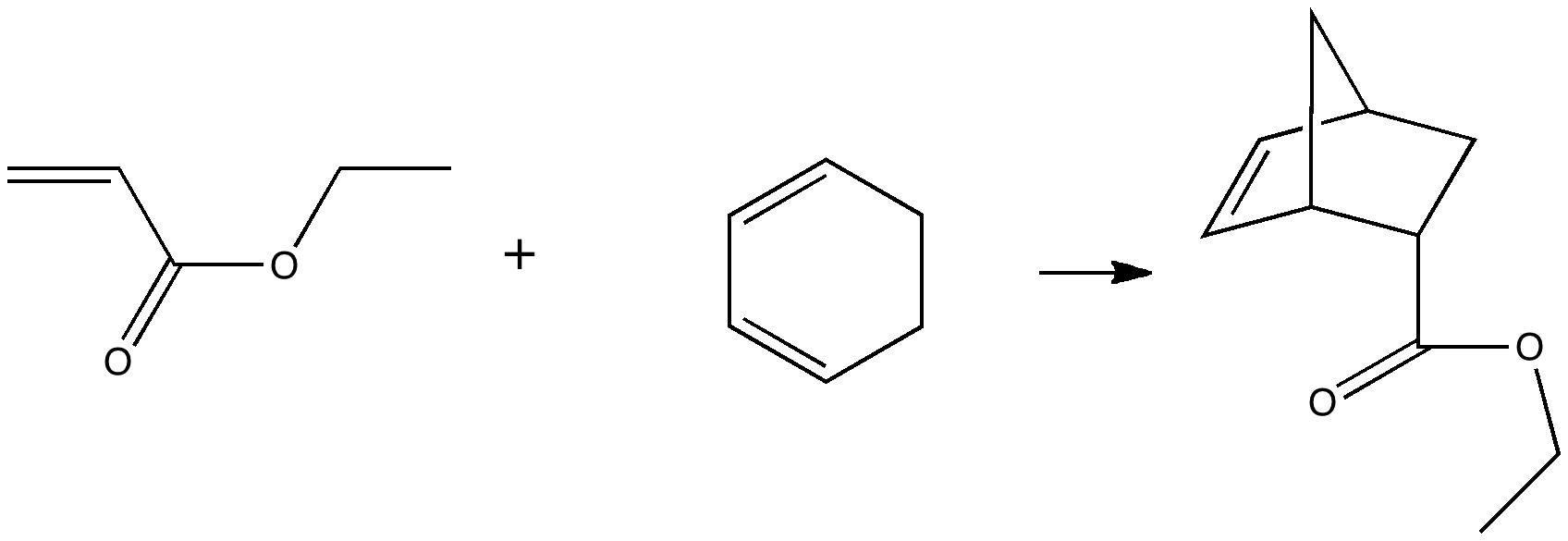
Reactions in cavities can adopt quite different characteristics from those in solvents. Thus first example of the catalysis of the Diels-Alder reaction inside an organic scaffold was reported by Endo, Koike, Sawaki, Hayashida, Masuda, and Aoyama[cite]10.1021/ja964198s[/cite], where the reaction shown below is speeded up very greatly in the presence of a crystalline lattice of the anthracene derivative shown below. A Diels-Alder reaction.