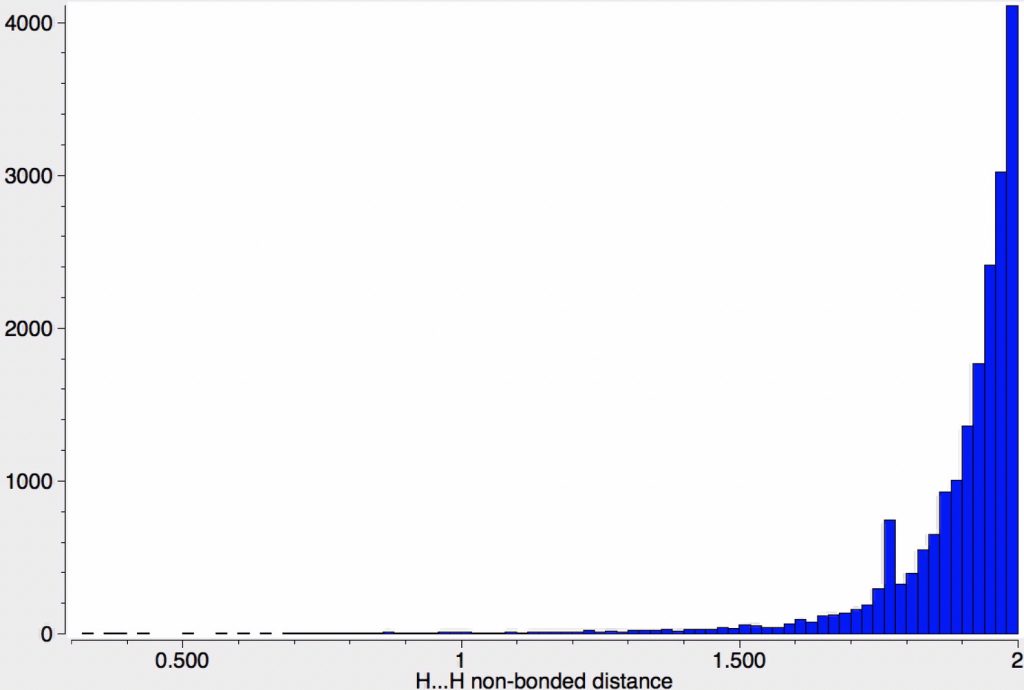
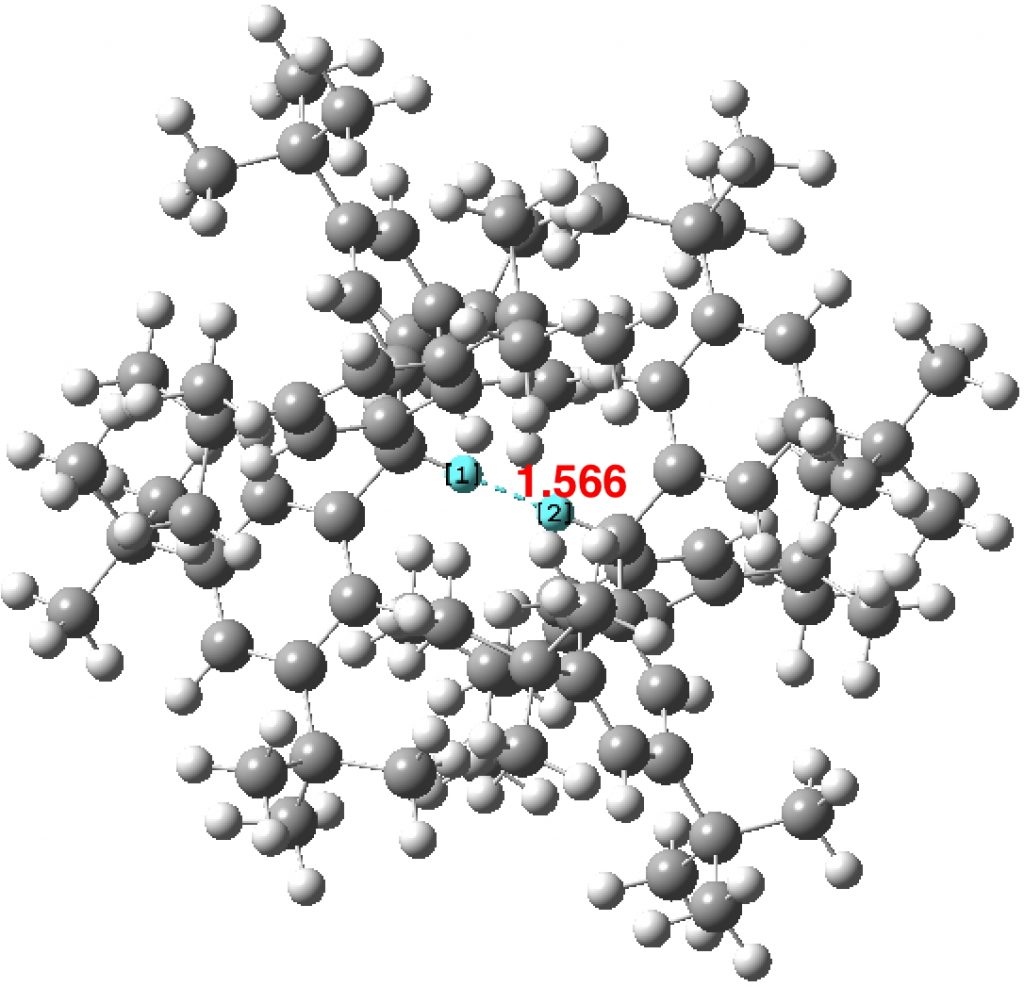
The triennial conference is this year located in Munich. With 1500 participants and six parallel sessions, this report can give only a flavour of proceedings. Edward Valeev talked about the scaling problem in coupled cluster theories, the so-called gold standard for computing the energy and properties of small molecules.