The butterfly effect summarises how a small change to a system may result in very large and often unpredictable (chaotic) consequences. If the system is merely on the edge of chaos, the consequences are predictable, but nevertheless finely poised between e.g. two possible outcomes. Here I ask how a molecule might manifest such behaviour. Two examples of the molecule above are known, differing only in the nature of the R group.
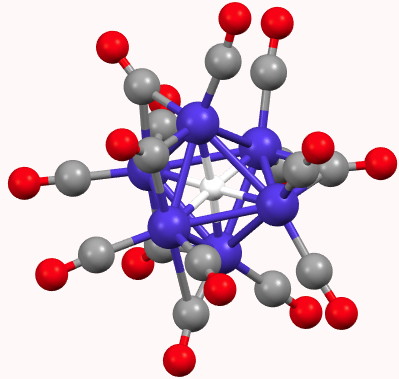
A feature of a blog which is quite different from a journal article is how rapidly a topic might evolve. Thus I started a few days ago with the theme of dicarbon (C 2 ), identifying a metal carbide that showed C 2 as a ligand, but which also entrapped a single carbon in hexa-coordinated mode.
A comment made on the previous post on the topic of hexa-coordinate carbon cited an article entitled “ Observation of hypervalent CLi 6 by Knudsen-effusion mass spectrometry “[cite]10.1038/355432a0[/cite] by Kudo as a amongst the earliest of evidence that such species can exist (in the gas phase). It was a spectacular vindication of the earlier theoretical
C 2 (dicarbon) is certainly interesting from a theoretical point of view. Whether or not it can be described as having a quadruple bond has induced much passionate discussion[cite]10.1038/nchem.1263[/cite],[cite]10.1002/anie.201208206[/cite],[cite]10.1002/anie.201301485[/cite],[cite]10.1002/anie.201302350[/cite]. Its occurrence in space and in flames is also well-known.
A reader asked me about the mechanism of the reaction of 2-picoline N-oxide with acetic anhydride to give 2-acetoxymethylpyridine (the Boekelheide Rearrangement[cite]10.1002/ejoc.201000936[/cite]). He wrote ” I don’t understand why the system should prefer to go via fragmentation-recombination (… the evidence being that oxygen labelling shows scrambling) when there is an easy concerted pathway available (… a
The title of this post comes from a comment posted by Ryan, who asks about isocyanide’s role (in the form of the anion of tosyl isocyanide, or TosMIC) in two named reactions, Van Leusen and Ugi FCR. “In Van Leusen, it (the isocyanide) acts as an electrophile: however, in Ugi, it acts as a nucleophile”. Here are some valence bond forms for this species;
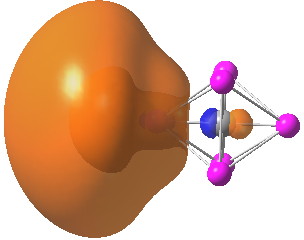
In the preceding post, I introduced Dewar’s π-complex theory for alkene-metal compounds, outlining the molecular orbital analysis he presented, in which the filled π-MO of the alkene donates into a Ag + empty metal orbital and back-donation occurs from a filled metal orbital into the alkene π* MO. Here I play a little “what if” game with this scenario to see what one can learn from doing so. Firstly, I will use
The period 1951–1954 was a golden one for structural chemistry; proteins, DNA, Ferrocene (1952) and the one I discuss here, a bonding model for Zeise’s salt ( 3 ). In “A review of π Complex Theory”, Bull. Soc. Chim. Fr. , 1951 , 1 8 , C79 (it is not online) M. J. S. Dewar sets out his theory of the role of π-complexes in (mostly) organic chemistry.
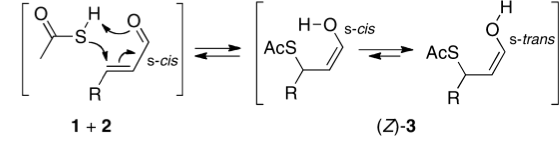
Lukas, who occasionally comments on this blog, sent me the following challenge. In a recent article[cite]10.1021/jo3021709[/cite] he had proposed that the stereochemical outcome ( Z ) of reaction between a butenal and thioacetic acid as shown below arose by an unusual concerted cycloaddtion involving an S-H bond.
In a previous post on the topic, I remarked how the regiospecific ethanolysis of propene epoxide[cite]10.1021/ja01208a047[/cite] could be quickly and simply rationalised by inspecting the localized NBO orbital calculated for either the neutral or the protonated epoxide. This is an application of Hammond’s postulate[[cite]10.1021/ja01607a027[/cite] in extrapolating the properties of a reactant to its reaction transition state.