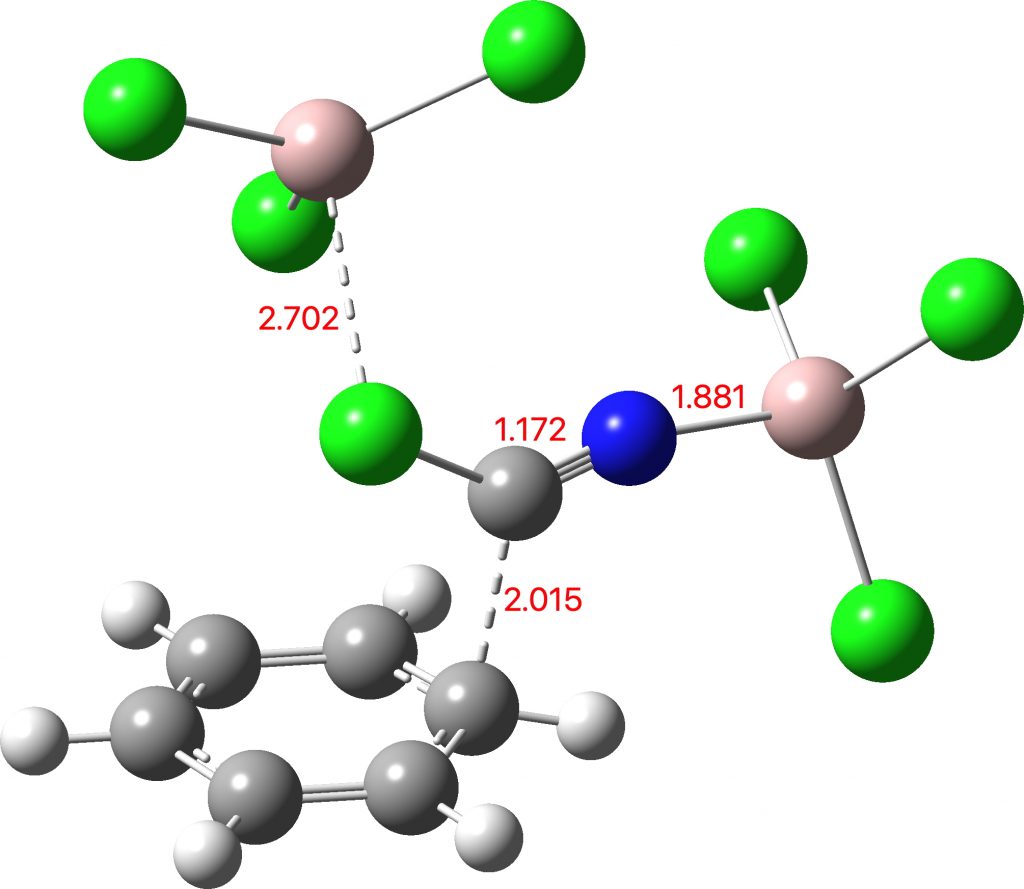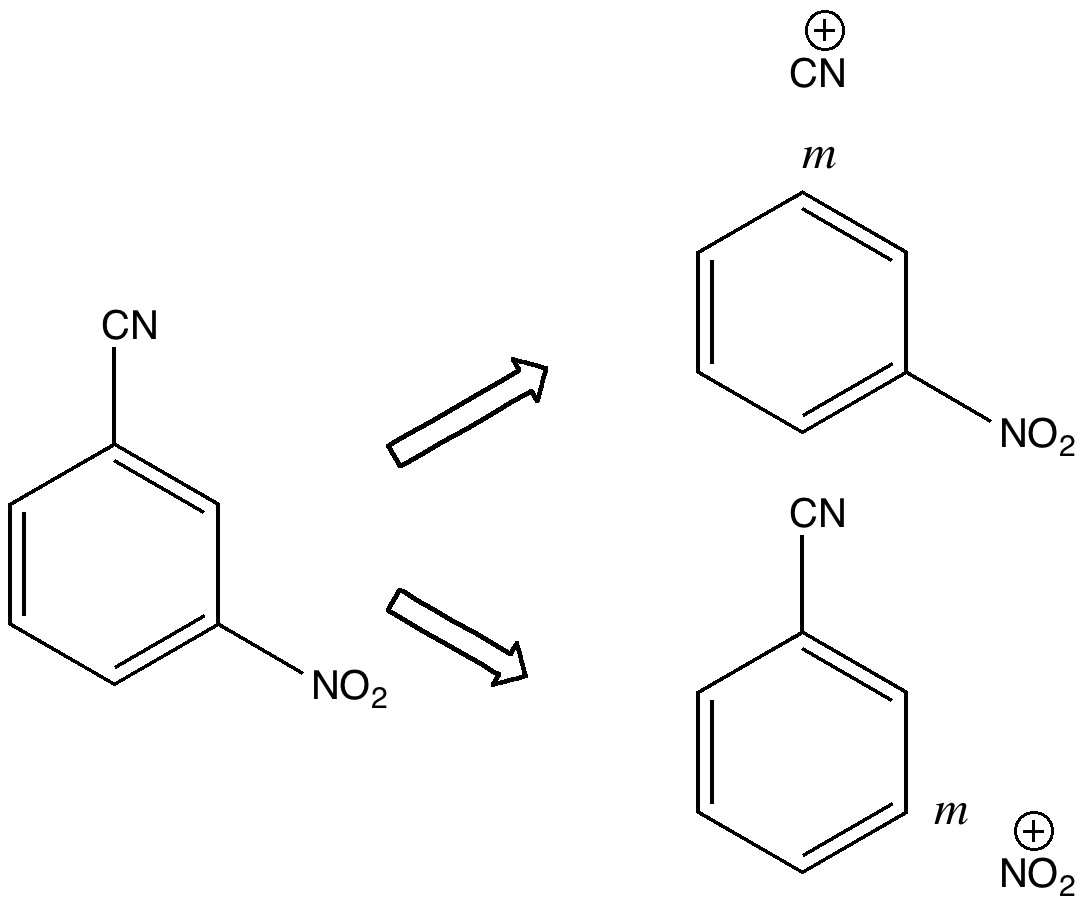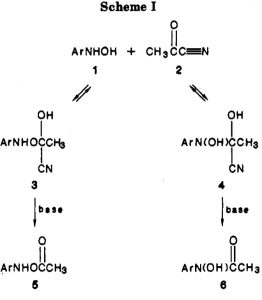
Minds (and memories) can work in wonderful ways. In 1987[cite]10.1021/jo00389a050[/cite] we were looking at the properties of “stable” tetrahedral intermediates formed in carbonyl group reactions. The reaction involved adding phenylhydroxylamine to acetyl cyanide.