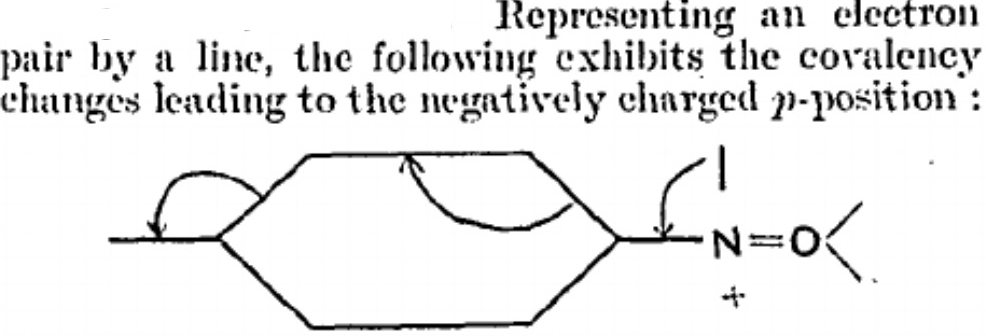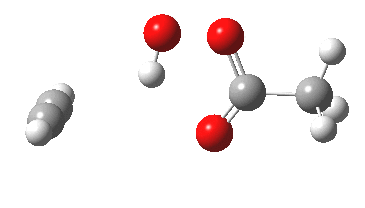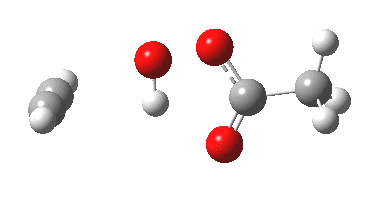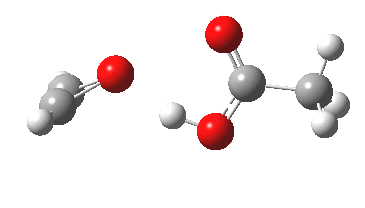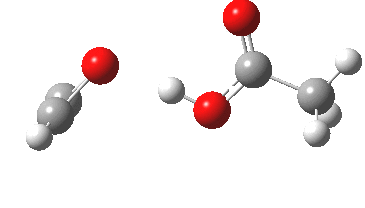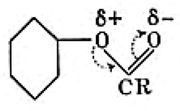
I have several times used arrow pushing on these blogs. But since the rules for this convention appear to be largely informal, and there appears to be no definitive statement of them, I thought I would try to produce this for our students. This effort is here shared on my blog. It is what I refer to as the standard version; an advanced version is in preparation. Such formality might come as a surprise to some;