The conformational analysis of cyclohexane is a mainstay of organic chemistry. Is there anything new that can be said about it? Let us start with the diagram below: This identifies the start of the process as a chair conformation of cyclohexane, with D 3d symmetry.
Years ago, I was travelling from Cambridge to London on a train. I found myself sitting next to a chemist, and (as chemists do), he scribbled the following on a piece of paper. When I got to work the next day Vera (my student) was unleashed on the problem, and our thoughts were published[cite]10.1039/C39920001323[/cite]. That was then. This is now.
The anti-periplanar principle permeates organic reactivity. Here I pick up on an example of the antiperiplanar E2 elimination (below, blue) by comparing it to a competing reaction involving a [1,2] antiperiplanar migration (red). The relative rates of these two processes will depend on several factors such as the ability of Cl to donate electrons (red) vs the basicity of the chloride anion (blue) and of course solvent polarity.
The previous post explored why E2 elimination reactions occur with an antiperiplanar geometry for the transition state. Here I have tweaked the initial reactant to make the overall reaction exothermic rather than endothermic as it was before. The change is startling. The exothermicity is of course due to the aromatisation of the ring. The IRC is however quite different from before. IRC for E2 elimination.
The so-called E2 elimination mechanism is another one of those mainstays of organic chemistry. It is important because it introduces the principle that anti-periplanarity of the reacting atoms is favoured over other orientations such as the syn-periplanar form; Barton used this principle to great effect in developing the theory of conformational analysis. Here I explore its origins.
The world’s smallest nano car was recently driven a distance of 6nm along a copper track. When I saw this, I thought it might be interesting to go under the hood and try to explain what makes its engine tick and its fuel work. The molecule above represents (I think) the essentials of the engine. Its resting geometry in the S 0 electronic state is shown below. The resting geometry of the engine.
My previous post introduced the interesting guts of taxol. Two different isomers can exist, and these are called atropisomers; one has the carbonyl group pointing up, the other down. The barrier to their interconversion in this case is generated by a rotation about the two single bonds connecting the carbonyl group to the rest of the molecule.
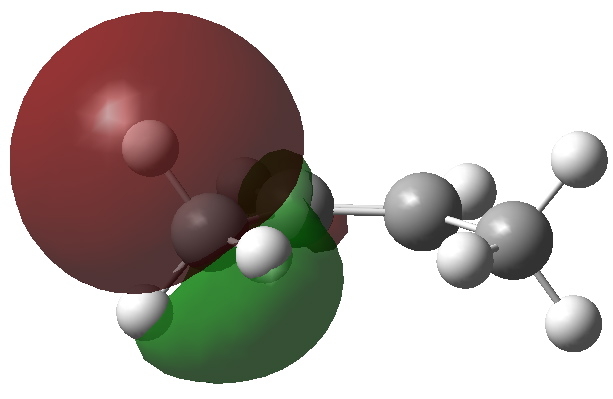
I wrote earlier about the strangely close contact between two hydrogen atoms in cis -butene. The topology of the electron density showed characteristics of a bond, but is it a consensual union?
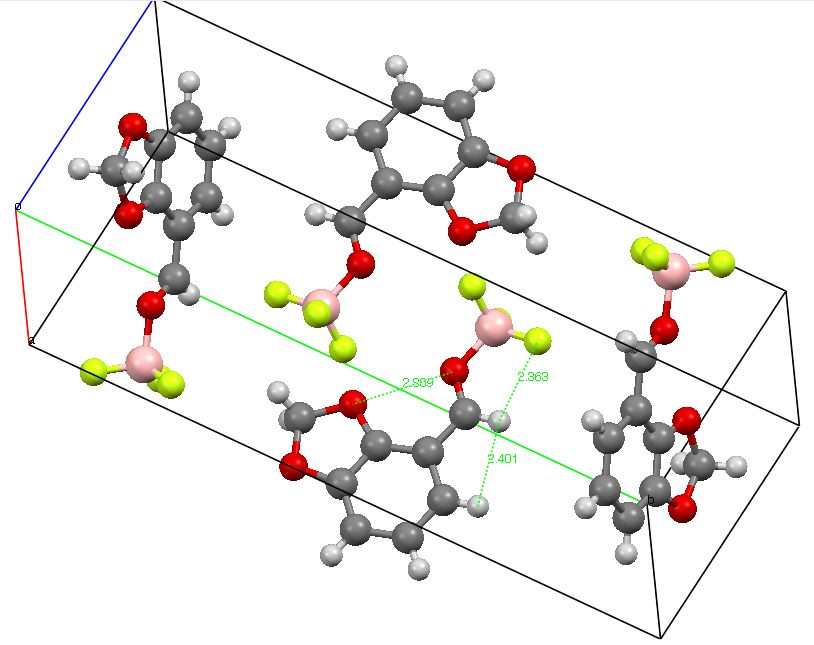
The title of this post paraphrases E. J. Corey’s article in 1997 (DOI: 10.1016/S0040-4039(96)02248-4) which probed the origins of conformation restriction in aldehydes. The proposal was of (then) unusual hydrogen bonding between the O=C-H…F-B groups. Here I explore whether the NCI (non-covalent-interaction) method can be used to cast light on this famous example of how unusual interactions might mediate selectivity in organic reactions.
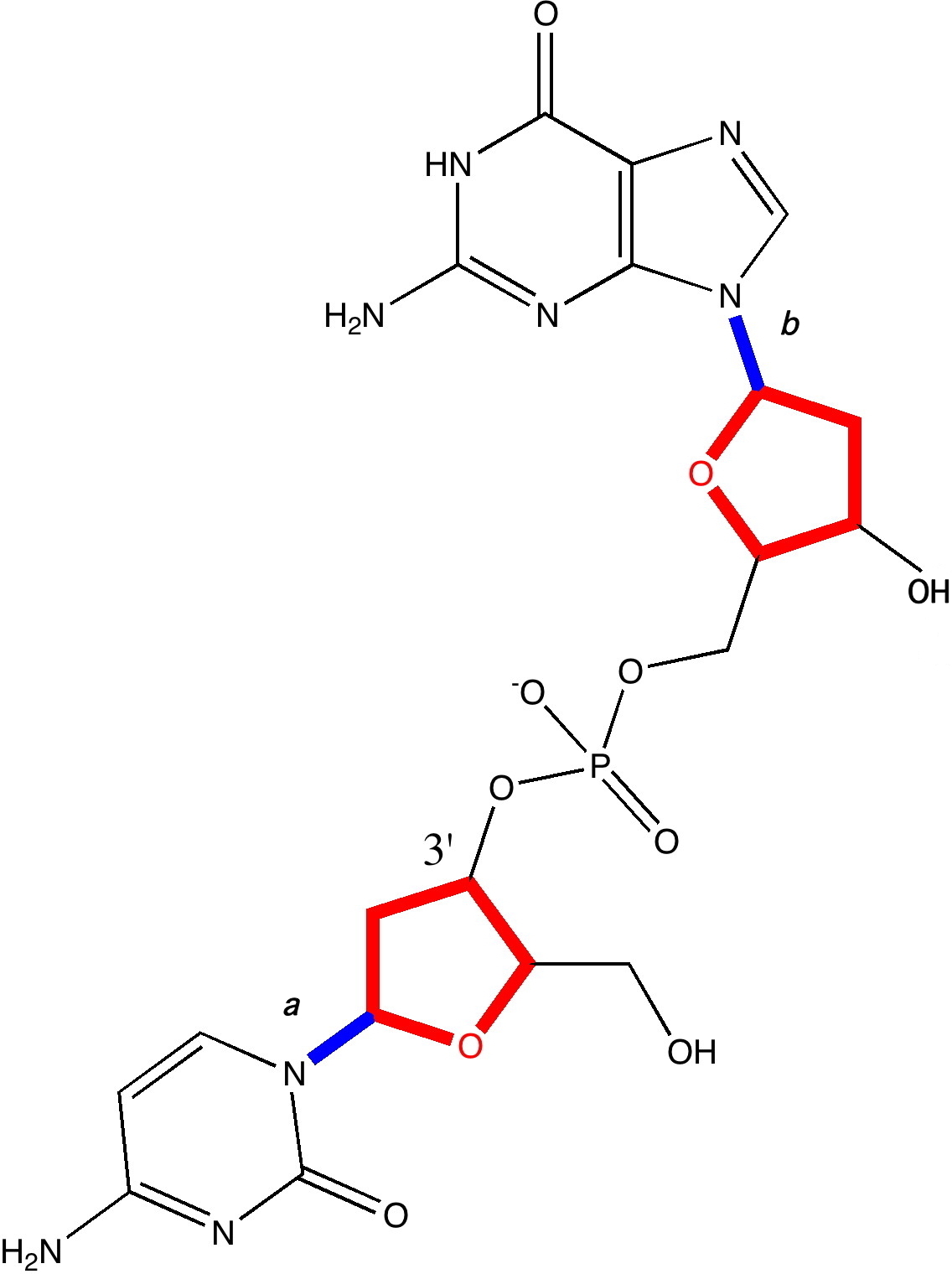
In earlier posts, I alluded to what might make DNA wind into a left or a right-handed helix. Here I switch the magnification of our structural microscope up a notch to take a look at some more inner secrets. A fragment of a single chain of DNA, taken from a Z-helix.