In several posts a year or so ago I considered various suggestions for the most polar neutral molecules, as measured by the dipole moment. A record had been claimed[cite]10.1002/anie.201508249[/cite] for a synthesized molecule of ~14.1±0.7D. I pushed this to a calculated 21.7D for an admittedly hypothetical and unsynthesized molecule.
C&EN has again run a vote for the 2017 Molecules of the year. Here I take a look not just at these molecules, but at how FAIR (Findable, Accessible, Interoperable and Reusable) the data associated with these molecules actually is. I went about finding out as follows: The article DOI for all seven candidates was linked to the C&EN site.
Another post inspired by a comment on an earlier one; I had been discussing compounds of the type I.I n (n=4,6) as possible candidates for hypervalency.
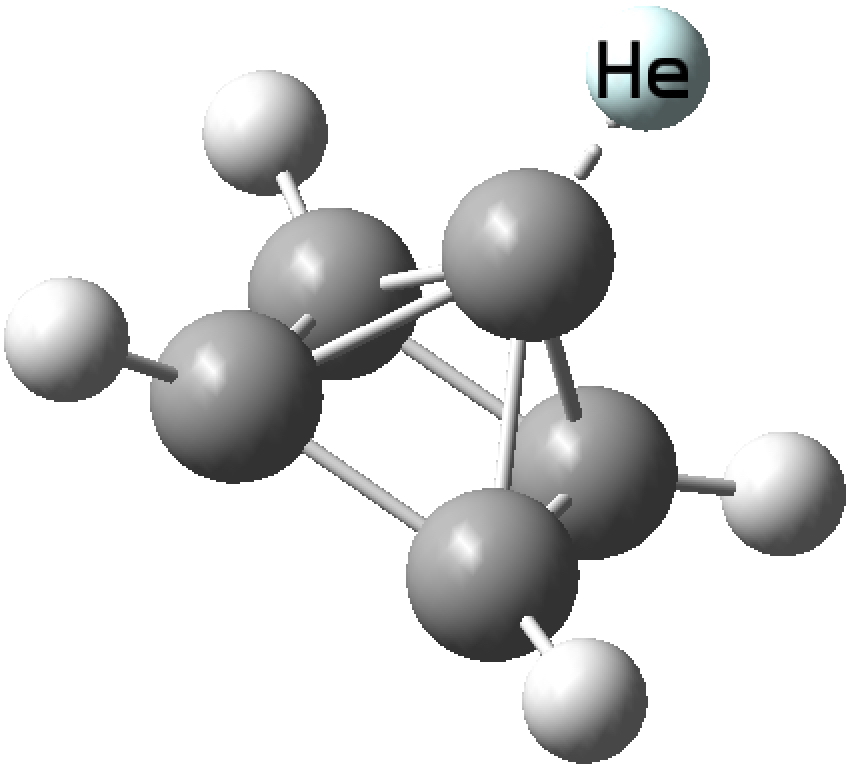
Last year, this article[cite]10.1038/nchem.2716[/cite] attracted a lot of attention as the first example of molecular helium in the form of Na 2 He. In fact, the helium in this species has a calculated ‡ bond index of only 0.15 and it is better classified as a sodium electride with the ionisation induced by pressure and the presence of helium atoms.
I don’t normally write about the pharmaceutical industry, but I was intrigued by several posts by Derek Lowe (who does cover this area) on the topic of creating new drugs by deuterating existing ones. Thus he covered the first deuterated drug receiving FDA approval last year, having first reviewed the concept back in 2009. So when someone introduced me to sila-haloperidol , I checked to see if Derek had written about it.
The title here is from an article on metalenses[cite]10.1021/acs.nanolett.6b01897[/cite] which caught my eye. Metalenses are planar and optically thin layers which can be manufactured using a single-step lithographic process. This contrasts with traditional lenses that are not flat and where the optical properties result from very accurately engineered curvatures, which in turn are expensive to manufacture.
Recollect the suggestion that diazomethane has hypervalent character[cite]10.1039/C5SC02076J[/cite]. When I looked into this, I came to the conclusion that it probably was mildly hypervalent, but on carbon and not nitrogen. Here I try some variations with substituents to see what light if any this casts.
Previously: “Non-polar” species such as SeMe6, SMe6, ClMe3, ClMe5 all revealed interesting properties for the Se-C, S-C or Cl-C “single” bonds. The latter two examples in particular hinted at internal structures for these single bonds, as manifested by two ELF basins for some of the bonds.
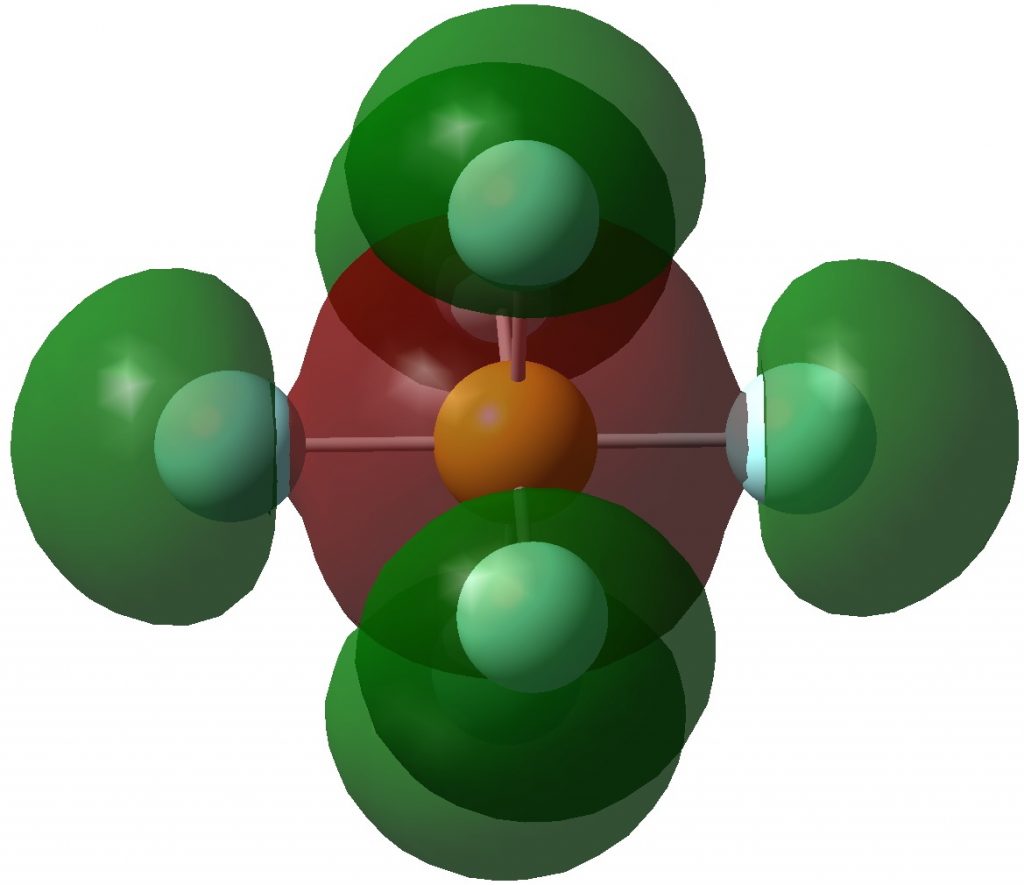
Following on from discussing octet expansion in species such as SeMe 6 , ClMe 3 and ClMe 5 , I felt impelled to return to SF 6 , often used as an icon for hypervalence.
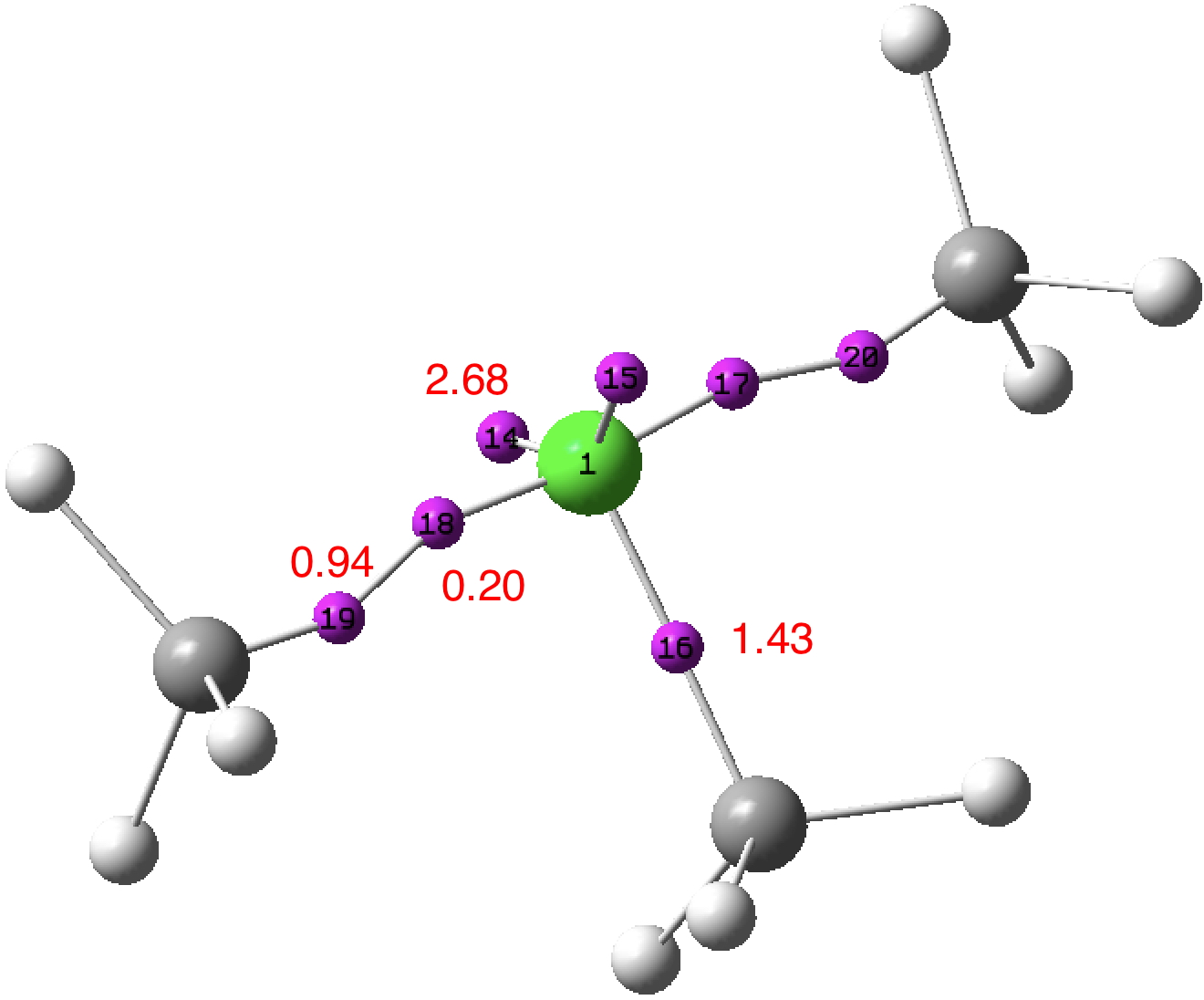
A few years back, I took a look at the valence-shell electron pair repulsion approach to the geometry of chlorine trifluoride, ClF 3 using so-called ELF basins to locate centroids for both the covalent F-Cl bond electrons and the chlorine lone-pair electrons.