Previously, I had noted that Corey reported in 1963/65 the total synthesis of the sesquiterpene dihydrocostunolide. Compound 16 , known as Eudesma-1,3-dien-6,13-olide was represented as shown below in black; the hydrogen shown in red was implicit in Corey’s representation, as was its stereochemistry. As of this instant, this compound is just one of 64,688,893 molecules recorded by Chemical Abstracts.
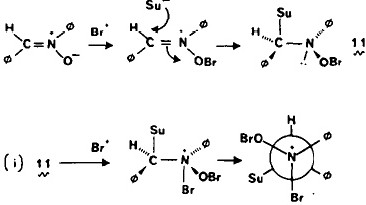
How one might go about answering the question: do alkenes promote anomeric effects? A search of chemical abstracts does not appear to cite any examples (I may have missed them of course, since it depends very much on the terminology you use, and new effects may not yet have any agreed terminology) and a recent excellent review of hyperconjugation does not mention it. Here I show how one might provide an answer.
Most of the chemical structure diagrams in this blog originate from Chemdraw, which seems to have been around since the dawn of personal computers! I have tended to use this program to produce JPG bitmaps for the blog, writing them out in 4x magnification, so that they can be scaled down for display whilst retaining some measure of higher resolution if needed for other purposes.
I have for perhaps the last 25 years been urging publishers to recognise how science publishing could and should change. My latest thoughts are published in an article entitled “ The past, present and future of Scientific discourse ” (DOI: 10.1186/1758-2946-3-46). Here I take two articles, one published 58 years ago and one published last year, and attempt to reinvent some aspects.
Bonds are a good example of something all chemists think they can recognise when they see them. But they are also remarkably dependent on context. We are running a molecular modelling course at the moment, and I found myself explaining to someone how very context-sensitive they can be. I thought it might be useful to collect my thoughts here.
Steve Jobs death on October 5th 2011 was followed by a remarkable number of tributes and reflections on the impact the company he founded has had on the world. Many of these tributes summarise the effect as a visionary disruption . Here I describe from my own perspective some of the disruptions to chemistry I experienced (for another commentary, see here). Chemical diagram, circa 1983.
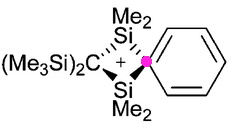
Charges in chemistry, like the grin on Lewis Carroll’s cat, can be mysterious creatures. Take for example the following structure, reported by Paul Lickiss and co-workers (DOI: 10.1039/b513203g). A silenium cation.
In 1986 or so, molecular modelling came of age. Richard Counts, who ran an organisation called QCPE (here I had already submitted several of the program codes I had worked on) had a few years before contacted me to ask for my help with his Roadshow. He had started these in the USA as a means of promoting QCPE, which was the then main repository of chemistry codes, and as a means of showing people how to use the codes.
As a personal retrospective of my use of computers (in chemistry), the Macintosh plays a subtle role. 1985: In the previous part, I noted how the Corvus Concept computer introduced a network hard drive (these still being too expensive for any one individual to afford one); the same principle applied to the 1985 Macintosh but now relating to the remarkable introduction of the laser printer.
Computers and I go back a while (44 years to be precise), and it struck me (with some horror) that I have been around them for ~62% of the modern computing era (Babbage notwithstanding, ~1940 is normally taken as the start of the modern computing era). So indulge me whilst I record this perspective from the viewpoint of the computers I have used over this 62% of the computing era.