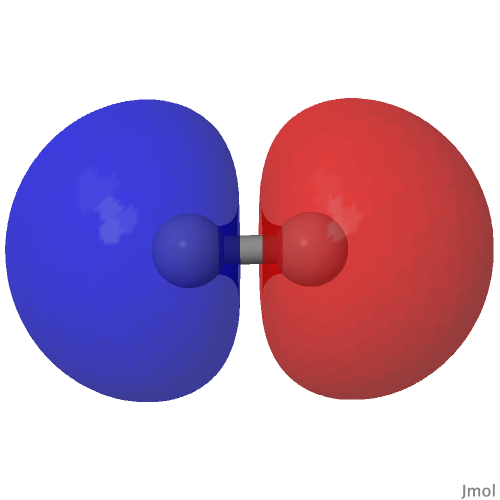
The classic anomeric effect operates across a carbon atom attached to oxygens. One (of the two) lone pairs on the oxygen can donate into the σ* orbital of the C-O of the other oxygen ( e.g. the red arrows) tending to weaken that bond whilst strengthening the donor oxygen C-O bond. Vice versa means e.g. the blue arrows weakening the other C-O bond.
From the last few posts here, you might have noticed much discussion about how the element carbon might sustain a quadruple bond. The original post on this topic from some years ago showed the molecular orbitals of the species CN + , which included two bonding π-types and a low lying nodeless bonding σ-orbital, all with double occupancies and adding up to a triple bond.
I noted in an earlier post the hypothesized example of (CO) 3 Fe⩸C[cite]10.1039/d0cp03436c[/cite] as exhibiting a carbon to iron quadruple bond and which might have precedent in known five-coordinate metal complexes where one of the ligands is a “carbide” or C ligand. I had previously mooted that the Fe⩸C combination might be replaceable by an isoelectronic Mn⩸N pair which could contain a quadruple bond to the nitrogen.
The proposed identification of molecules with potential metal to carbon quadruple bonds, in which the metal exhibits trigonal bipyramidal coordination rather than the tetrahedral modes which have been proposed in the literature[cite]10.1039/d0cp03436c[/cite],[cite]10.1039/d1cp00598g[/cite],[cite]10.1021/acs.jpclett.9b03484[/cite] leads on to asking whether simple trigonal coordination at the metal can also sustain this theme?
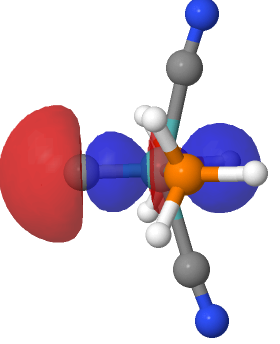
Introductory chemistry will tell us that a triple bond between say two carbon atoms comprises just one bond of σ-axial symmetry and two of π-symmetry. Increasingly mentioned nowadays is the possibility of a quadruple bond between carbon and either itself or a transition metal, as discussed in the previous post. Such a bond comprises TWO bonds of σ-axial symmetry.
Following from much discussion over the last decade about the nature of C 2 , a diatomic molecule which some have suggested sustains a quadruple bond between the two carbon atoms, new ideas are now appearing for molecules in which such a bond may also exist between carbon and a transition metal atom.
In the preceding post, I looked at a computed mechanism for the hydrolysis of a ketal by water. Of course, pure water consists of three potential catalysts, water itself or [H 2 O], and the products of autoionisation, [OH – ] and [H 3 O + ]. The latter are in much smaller concentration, equivalent to a penalty of ~11.9 kcal/mol on any free energy barrier.
The previous post was about an insecticide and made a point that the persistence of both insecticides and herbicides is an important aspect of their environmental properties. Water hydrolysis will degrade them, a typical residency time being in the order of a few days. I noted in passing a dioxepin-based herbicide[cite]10.1039/P29890001265[/cite] which contains a ketal motif and which in water can hydrolise to a ketone and alcohol.
Deltamethin is a pyrethroid insecticide for control of malaria which has been used for a little while. Perhaps inevitably, mosquitoes are developing resistance to it. So what could be done about countering this? Well, perhaps surprisingly, form a polymorph![cite]10.1073/pnas.2013390117[/cite] These crystal structure isomers are often highly undesirable;
For obvious reasons, anti-viral molecules are very much in the news at the moment. Thus Derek Lowe highlights Molnupiravir which is shown as a hydroxylamine, the representation originating from the Wikipedia page on the molecule. I like stereocentres more clearly identified using eg R / S notation and so I went to another source of information, SciFinder, which represents the molecule in a different way.