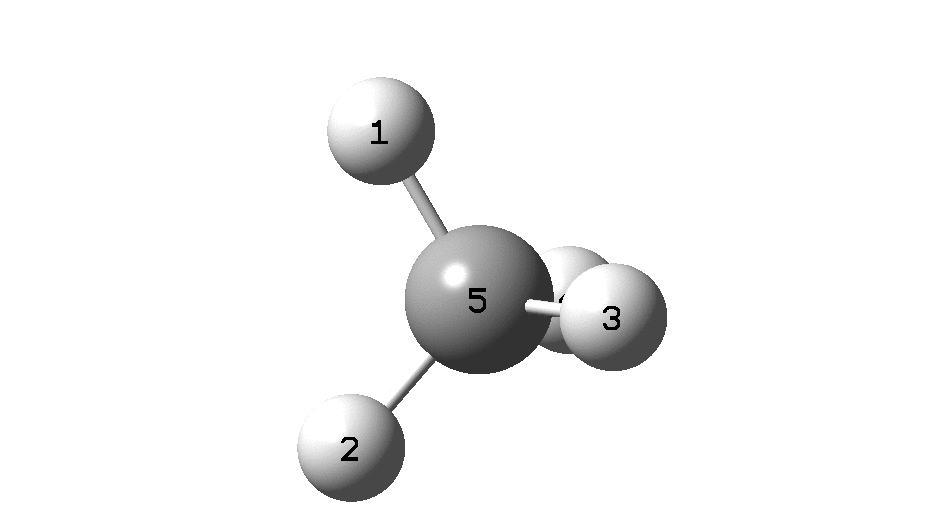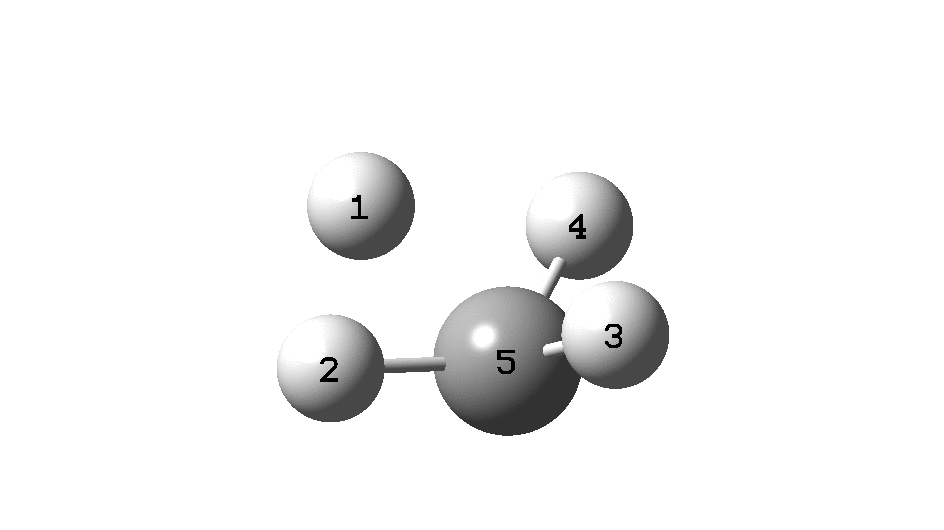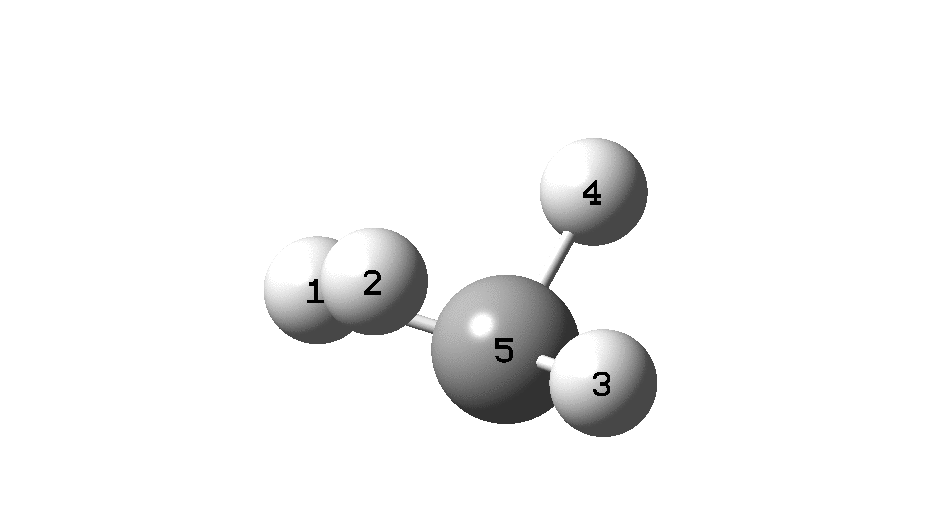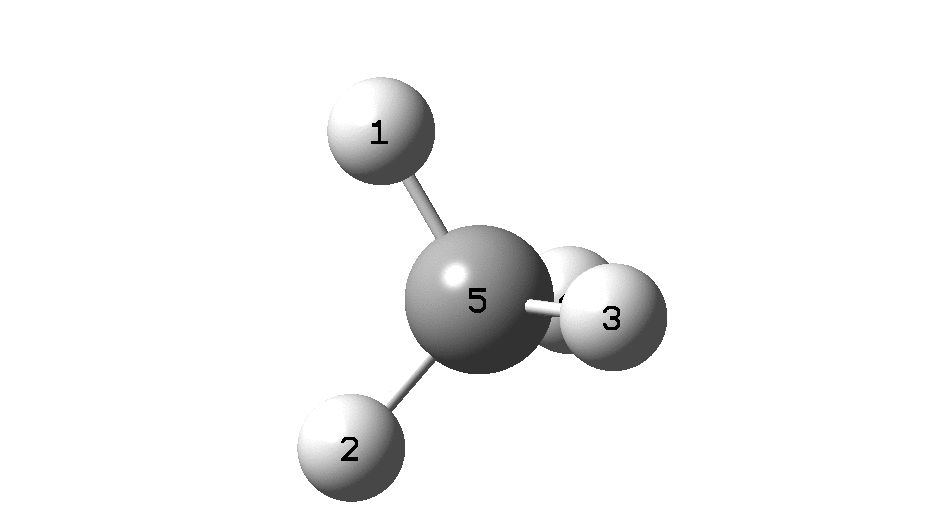Published
Author Henry Rzepa
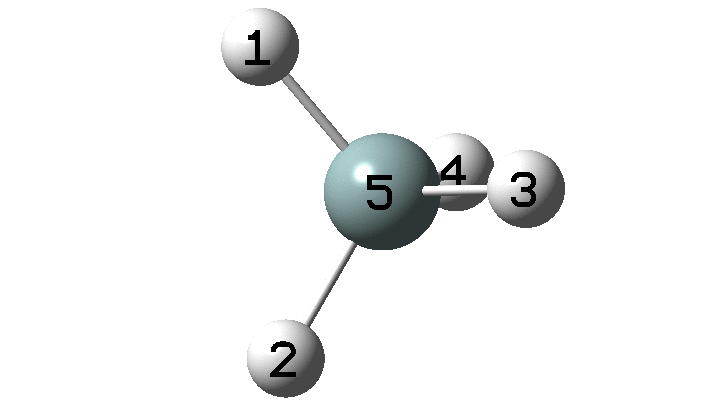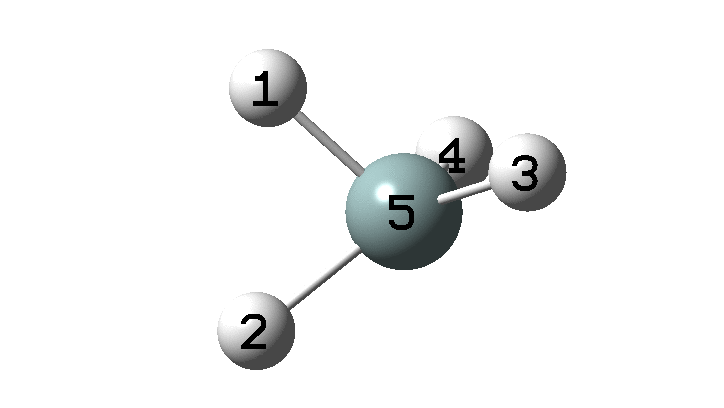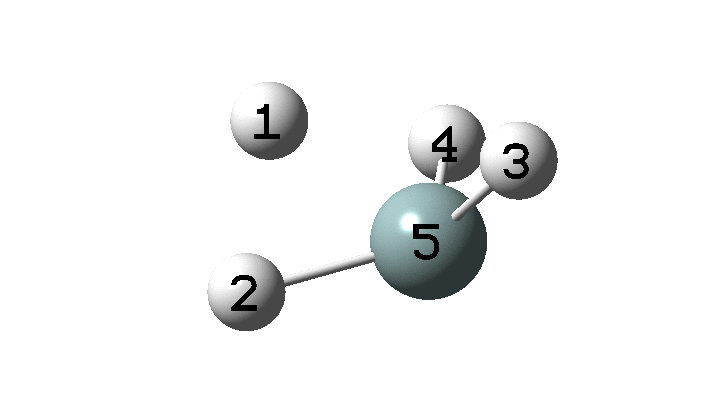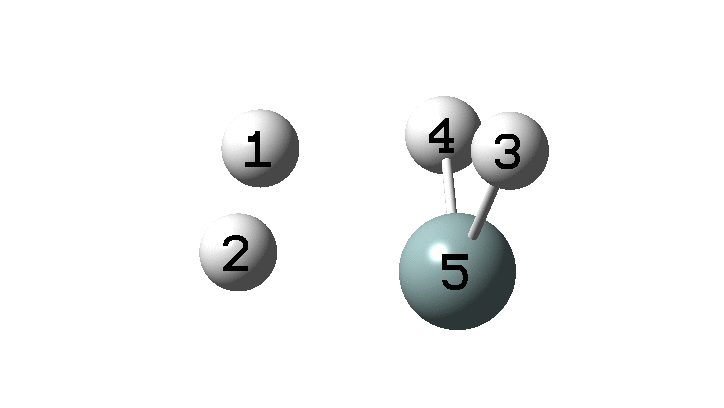
In the previous post, I found intriguing the mechanism by which methane (CH 4 ) inverts by transposing two of its hydrogens. Here I take a look at silane, SiH 4 . It appears it is a three-stage process! Firstly, silane eliminates molecular hydrogen to form a molecular complex between H 2 and SiH 2 (DOI: 10.14469/hpc/2290). The barrier (~60 kcal/mol) is very much lower than with methane.