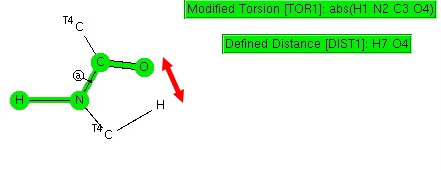
Amides with an H-N group are a component of the peptide linkage (O=C-NH). Here I ask what the conformation (it could also be called a configuration) about the C-N bond is. A search of the following type can be defined: The dihedral shown is for H-N-C=O (but this is equivalent to the C-C-N-C dihedral, which is also often called the dihedral angle associated with the peptide group). I have also added a distance, from a C-H to the

