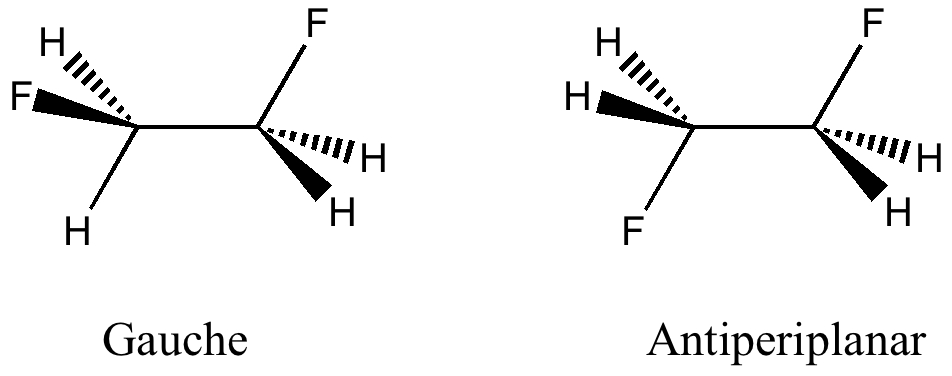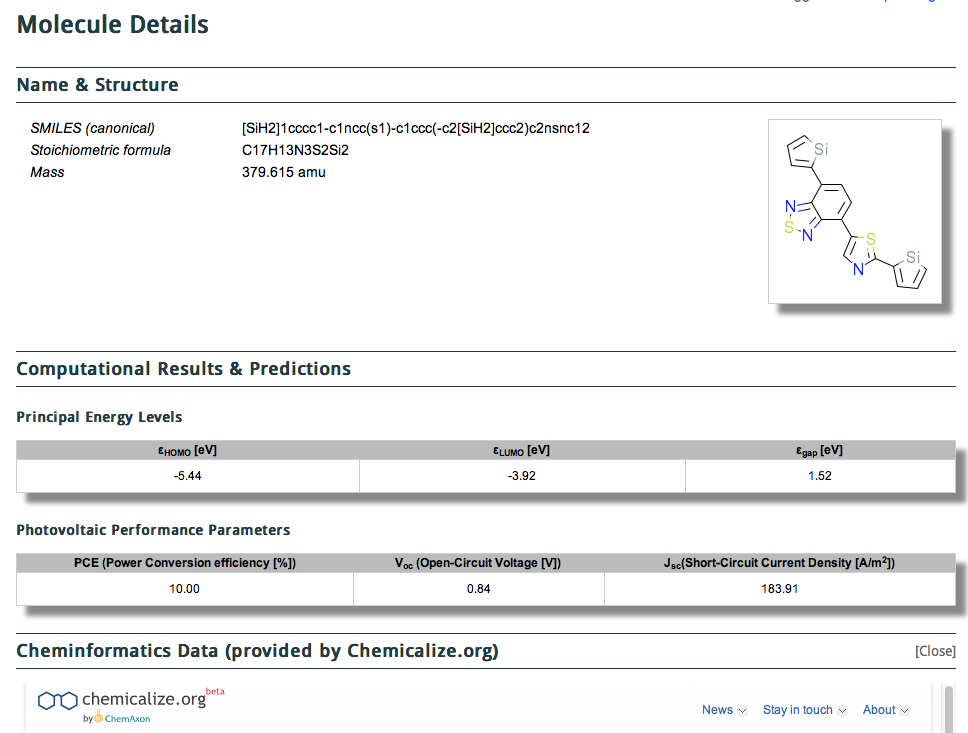
The title of this post summarises the contents of a new molecular database: www.molecularspace.org[cite]10.1021/jz200866s[/cite] and I picked up on it by following the post by Jan Jensen at www.compchemhighlights.org (a wonderful overlay journal that tracks recent interesting articles). The molecularspace project more formally is called “ The Harvard Clean Energy Project: Large-scale computational screening and design of organic