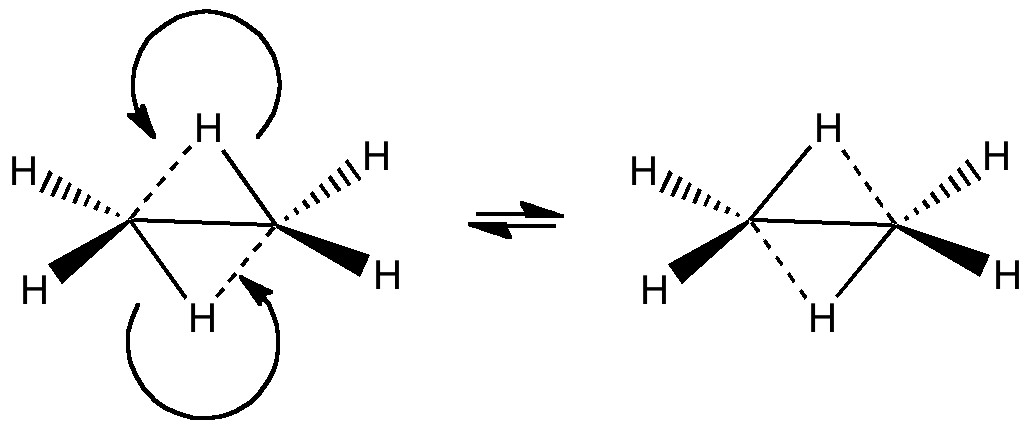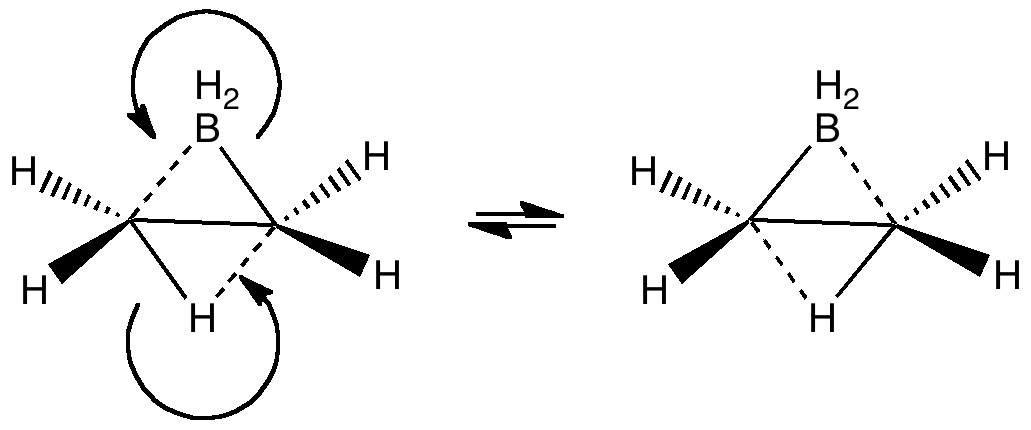
The last two posts have played a game of find the electrons. We saw how the dyotropic rearrangement of ethane borrowed electrons from the C-C bond, and how 1,2,dibromoethane went ionic on us. How about this mixed system, in which a hydrogen and a BH 2 swap their positions? Dyotropic rearrangement involving boron and hydrogen. It is yet again different.