Ken Houk’s group has recently published this study of cycloaddition reactions, using a combination of classical transition state location followed by molecular dynamics trajectory calculations,[cite]10.1021/jacs.8b12674[/cite] and to which Steve Bachrach’s blog alerted me. The reaction struck me as being quite polar (with cyano groups) and so I took a look at the article to see what both the original[cite]10.1021/jo00042a039[/cite] experimental
The example a few posts back of how methane might invert its configuration by transposing two hydrogen atoms illustrated the reaction mechanism by locating a transition state and following it down in energy using an intrinsic reaction coordinate (IRC). Here I explore an alternative method based instead on computing a molecular dynamics trajectory (MD). I have used ethane instead of methane, since it is now possible to

This post is prompted by the appearance of a retrospective special issue of C&E news, with what appears to be its very own Website: internet.cenmag.org.
I blogged about this two years ago and thought a brief update might be in order now. To support the discussions here, I often perform calculations, and most of these are then deposited into a DSpace digital repository, along with metadata. Anyone wishing to have the full details of any calculation can retrieve these from the repository. Now in 2012, such repositories are more important than ever.
Twenty years are acknowledged to be a long time in Internet/Web terms. In the early days (in 1994), it was a taken that the passage of 1 Web day in the Internet time-warp was ~≡ 7 for the rest of the world (the same factor as applied to the lives of canines). This temporal warping can also be said to apply to computational chemistry.
During the 1960s, a holy grail of synthetic chemists was to devise an efficient route to steroids. R. B. Woodward was one the chemists who undertook this challenge, starting from compounds known as dienones ( e.g. 1 ) and their mysterious conversion to phenols ( e.g. 2 or 3 ) under acidic conditions.

I wrote in an earlier post how Pauling’s Nobel prize-winning suggestion in February 1951 of a (left-handed) α-helical structure for proteins[cite]10.1073/pnas.37.4.205[/cite] was based on the wrong absolute configuration of the amino acids (hence his helix should really have been the right-handed enantiomer). This was most famously established a few months later by Bijvoet’s[cite]10.1038/168271a0[/cite] definitive crystallographic
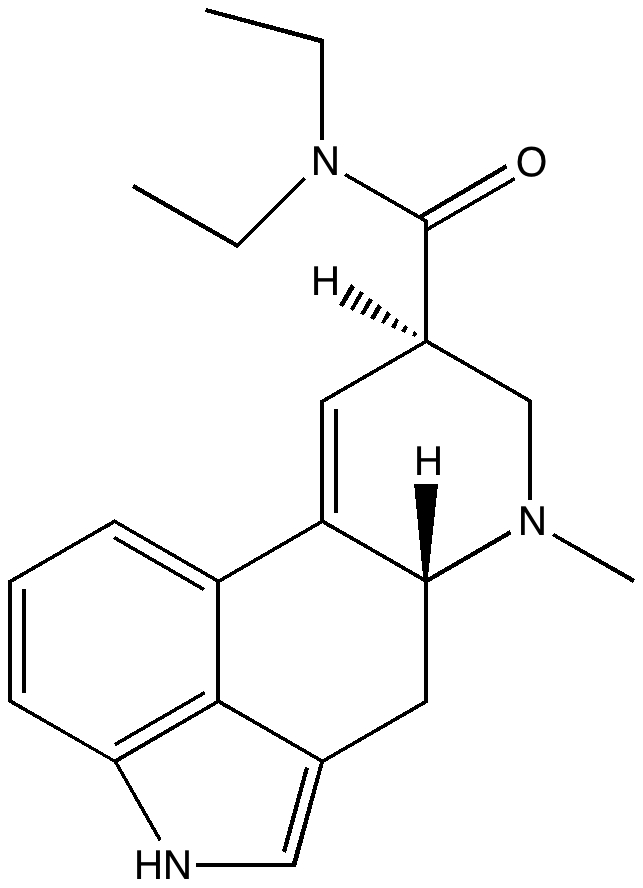
Moore’s law describes a long-term trend in the evolution of computing hardware, and it is often interpreted in terms of processing speed. Here I chart this rise in terms of the size of computable molecules. By computable I mean specifically how long it takes to predict the geometry of a given molecule using a quantum mechanical procedure. LSD, the 1975 benchmark for computable molecules.
