Ken Houk’s group has recently published this study of cycloaddition reactions, using a combination of classical transition state location followed by molecular dynamics trajectory calculations,[cite]10.1021/jacs.8b12674[/cite] and to which Steve Bachrach’s blog alerted me. The reaction struck me as being quite polar (with cyano groups) and so I took a look at the article to see what both the original[cite]10.1021/jo00042a039[/cite] experimental
The topic of this post originates from a recent article which is attracting much attention.[cite]10.1038/s41586-019-1059-9[/cite] The technique uses confined light to both increase the spatial resolution by around three orders of magnitude and also to amplify the signal from individual molecules to the point it can be recorded. To me, Figure 3 in this article summarises it nicely (caption: visualization of vibrational normal modes ).
There is a predilection amongst chemists for collecting records; one common theme is the length of particular bonds, either the shortest or the longest.
Five years back, I speculated about the mechanism of the epoxidation of ethene by a peracid, concluding that kinetic isotope effects provided interesting evidence that this mechanism is highly asynchronous and involves a so-called “hidden intermediate”. Here I revisit this reaction in which a small change is applied to the atoms involved. Below are two representations of the mechanism.
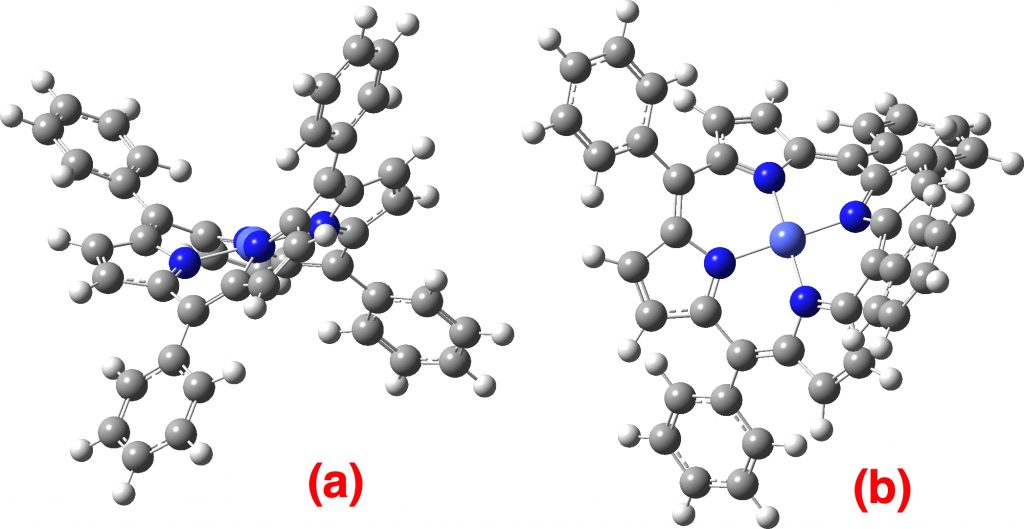
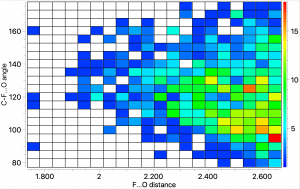
Recently, the 100th anniversary of the birth of the famous chemist Derek Barton was celebrated with a symposium. One of the many wonderful talks presented was by Tobias Ritter and entitled “ Late-stage fluorination for PET imaging ” and this resonated for me. The challenge is how to produce C-F bonds under mild conditions quickly so that 18 F-labelled substrates can be injected for the PET imaging.
In the previous post, I investigated the mechanism of cyclopropanation of an enal using a benzylic chloride using a quantum chemistry based procedure.

Following the general recognition of carbon as being tetrahedrally tetravalent in 1869 (Paterno) and 1874 (Van’t Hoff and Le Bell), an early seminal exploitation of this to the conformation of cyclohexane was by Hermann Sachse in 1890.[cite]10.1002/cber.189002301216 [/cite] This was verified when the Braggs in 1913[cite]10.1098/rspa.1913.0084[/cite], followed by an oft-cited article by Mohr in 1918,[cite]10.1002/prac.19180980123[/cite]
It was about a year ago that I came across a profusion of colour in my local Park. Although colour in fact was the topic that sparked my interest in chemistry many years ago (the fantastic reds produced by diazocoupling reactions), I had never really tracked down the origin of colours in many flowers. It is of course a vast field.
The molecules below were discussed in the previous post as examples of highly polar but formally neutral molecules, a property induced by aromatisation of up to three rings. Since e.g. compound 3 is known only in its protonated phenolic form, here I take a look at the basicity of the oxygen in these systems to see if deprotonation of the ionic phenol form to the neutral polar form is viable.
