There is a predilection amongst chemists for collecting records; one common theme is the length of particular bonds, either the shortest or the longest.

There is a predilection amongst chemists for collecting records; one common theme is the length of particular bonds, either the shortest or the longest.
A bond index (BI) approximately measures the totals of the bond orders at any given atom in a molecule. Here I ponder what the maximum values might be for elements with filled valence shells.
Previously: “Non-polar” species such as SeMe6, SMe6, ClMe3, ClMe5 all revealed interesting properties for the Se-C, S-C or Cl-C “single” bonds. The latter two examples in particular hinted at internal structures for these single bonds, as manifested by two ELF basins for some of the bonds.
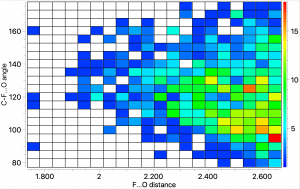
A few years back, I took a look at the valence-shell electron pair repulsion approach to the geometry of chlorine trifluoride, ClF 3 using so-called ELF basins to locate centroids for both the covalent F-Cl bond electrons and the chlorine lone-pair electrons.
An N-B single bond is iso-electronic to a C-C single bond, as per below. So here is a simple question: what form does the distribution of the lengths of these two bonds take, as obtained from crystal structures? The Conquest search query is very simple (no disorder, no errors). When applied to the Cambridge structure database (CSD) the following two distributions are obtained.

Early in 2011, I wrote about how the diatomic molecule Be 2 might be persuaded to improve upon its normal unbound state (bond order ~zero) by a double electronic excitation to a strongly bound species.
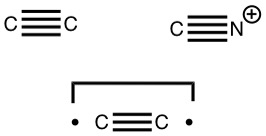
The chemical bond zoo is relatively small (the bond being a somewhat fuzzy concept, I am not sure there is an actual count of occupants). So when two new candidates come along, it is worth taking notice. I have previously noted the Chemical Bonds at the 21st Century-2017: CB2017 Aachen conference, where both were discussed.
Enols are simple compounds with an OH group as a substituent on a C=C double bond and with a very distinct conformational preference for the OH group. Here I take a look at this preference as revealed by crystal structures, with the theoretical explanation.
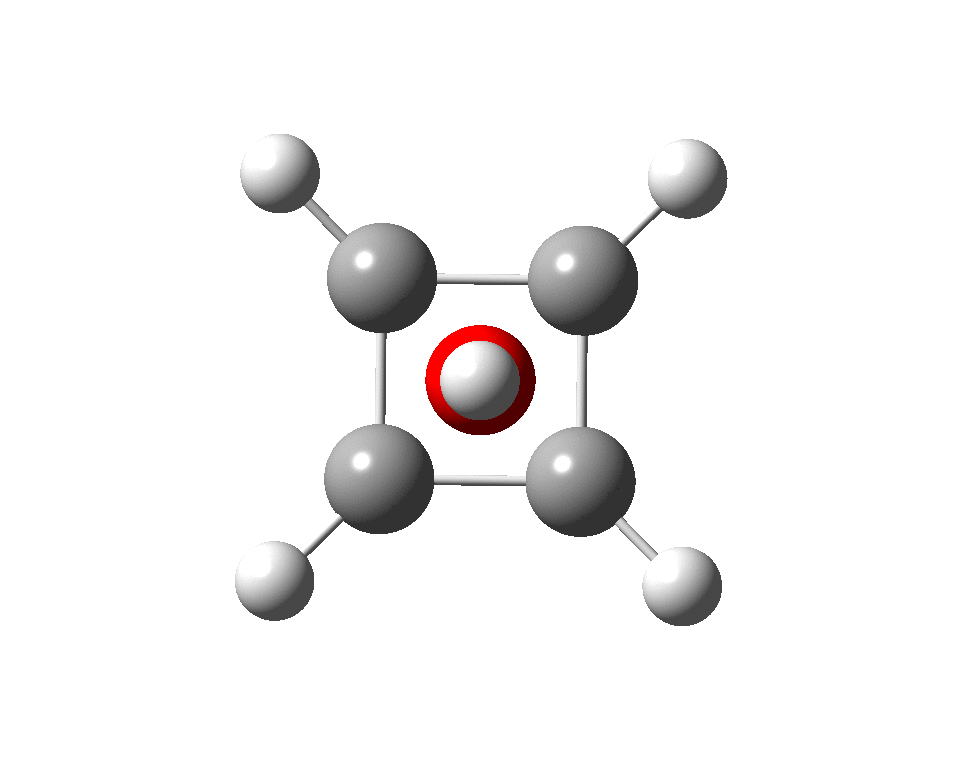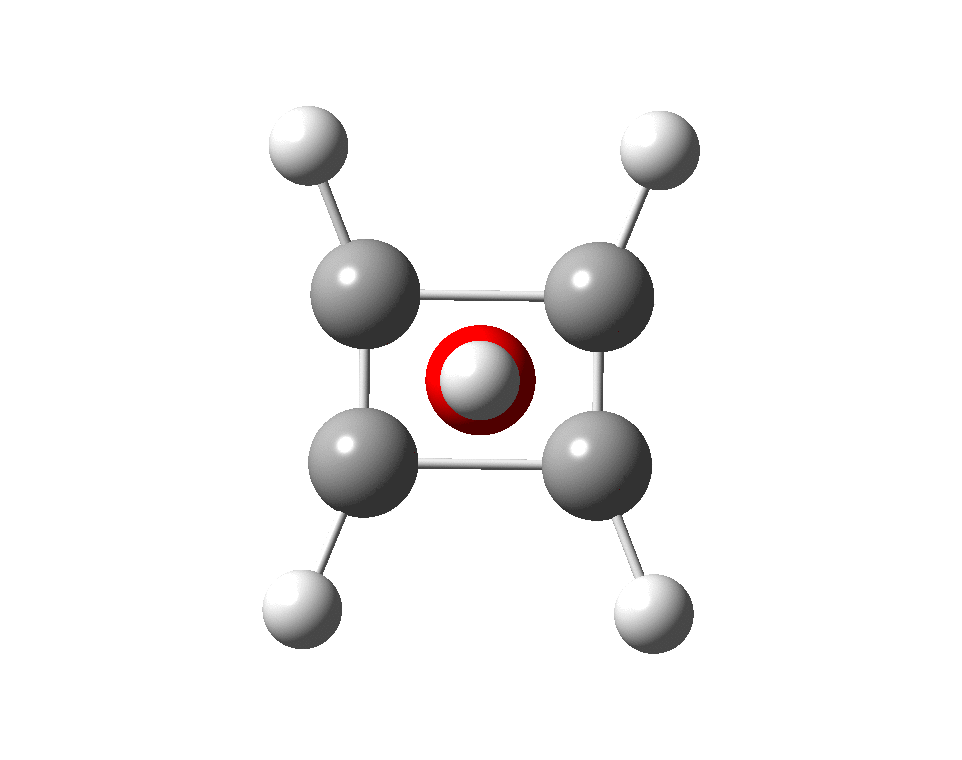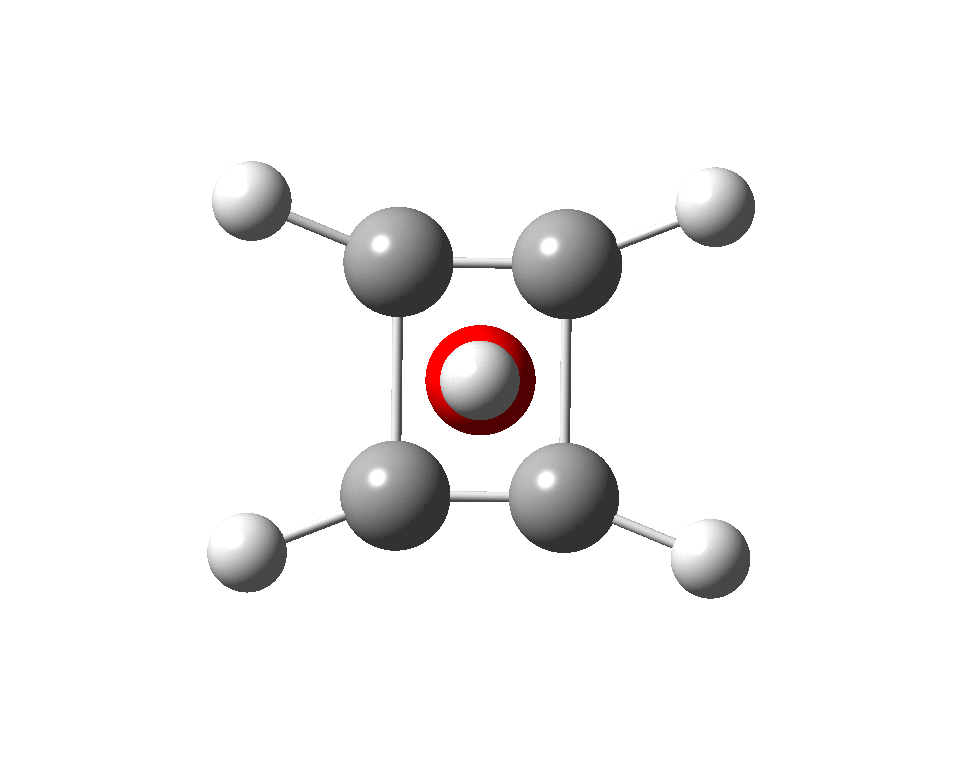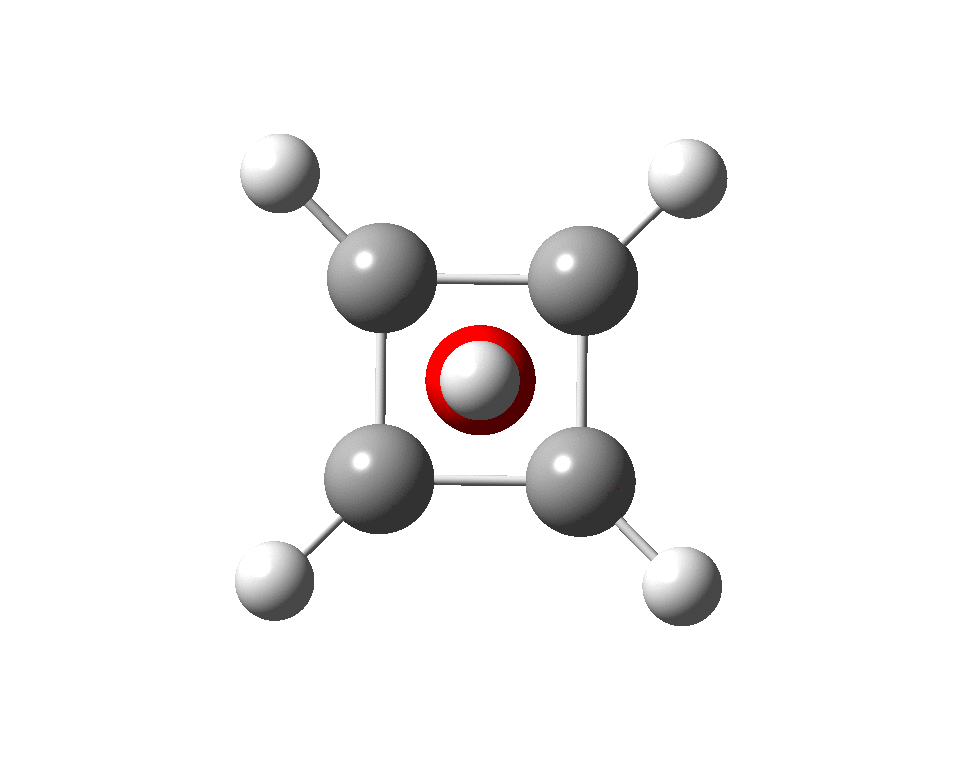
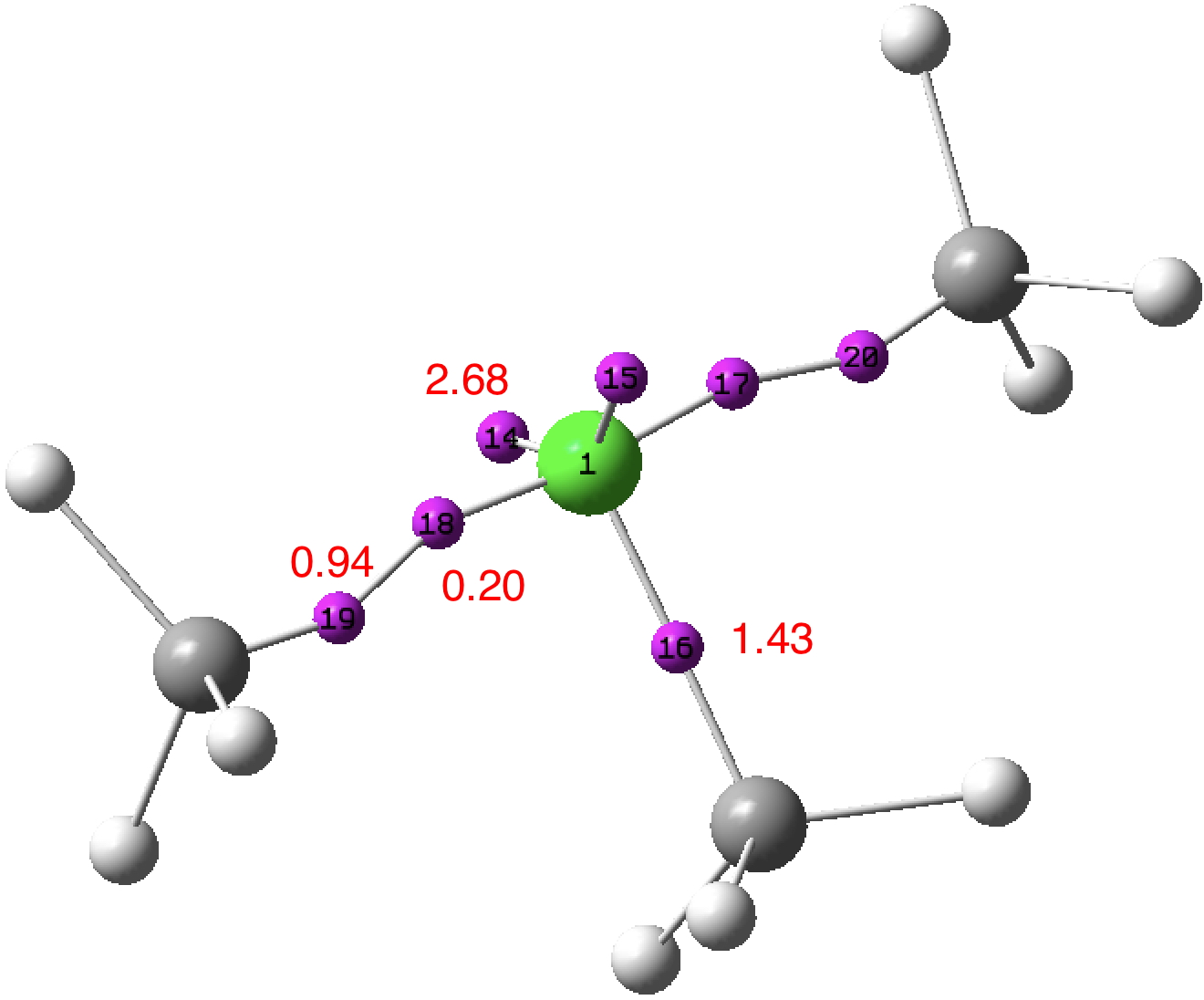
The previous post demonstrated the simple iso-electronic progression from six-coordinate carbon to five coordinate nitrogen. Here, a further progression to oxygen is investigated computationally. The systems are formally constructed from a cyclobutadienyl di-anion and firstly the HO 5+ cation, giving a tri-cationic complex. There are no examples of the resulting motif in the Cambridge structure database.
George Olah passed away on March 8th. He was part of the generation of scientists in the post-war 1950s who had access to chemical instrumentation that truly revolutionised chemistry.