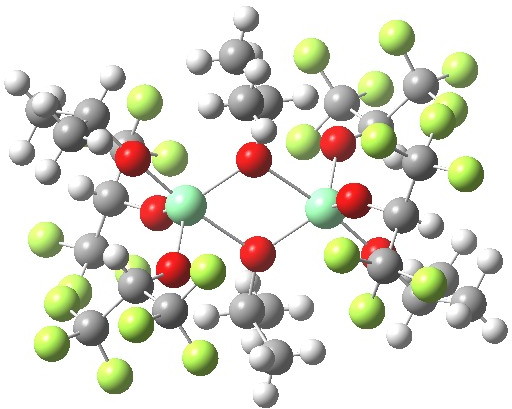
The iron complex shown below forms the basis for many catalysts.[cite]10.1002/anie.200502985[/cite] With iron, the catalytic behaviour very much depends on the spin-state of the molecule, which for the below can be either high (hextet) or medium (quartet) spin, with a possibility also of a low spin (doublet) state. Here I explore whether structural information in crystal structures can reflect such spin states.
I return to this reaction one more time. Trying to explain why it is enantioselective for the epoxide product poses peculiar difficulties. Most of the substituents can adopt one of several conformations, and some exploration of this conformational space is needed. Amongst the conformational possibilities are the two rotations shown below.
tpap[cite]10.1055%2Fs-1994-25538[/cite], as it is affectionately known, is a ruthenium-based oxidant of primary alcohols to aldehydes discovered by Griffith and Ley.
The Sharpless epoxidation of an allylic alcohol had a big impact on synthetic chemistry when it was introduced in the 1980s, and led the way for the discovery (design?) of many new asymmetric catalytic systems. Each achieves its chiral magic by control of the geometry at the transition state for the reaction, and the stabilizations (or destabilizations) that occur at that geometry.
Part one on this topic showed how a quantum mechanical model employing just one titanium centre was not successful in predicting the stereochemical outcome of the Sharpless asymmetric epoxidation.
Sharpless epoxidation converts a prochiral allylic alcohol into the corresponding chiral epoxide with > 90% enantiomeric excess[cite]10.1021/jo00369a032[/cite],[cite]10.1021/jo00360a058[/cite]. Here is the first step in trying to explain how this magic is achieved.
Astronomers who discover an asteroid get to name it, mathematicians have theorems named after them. Synthetic chemists get to name molecules (Hector’s base and Meldrum’s acid spring to mind) and reactions between them. What do computational chemists get to name? Transition states! One of the most famous of recent years is the Houk-List.
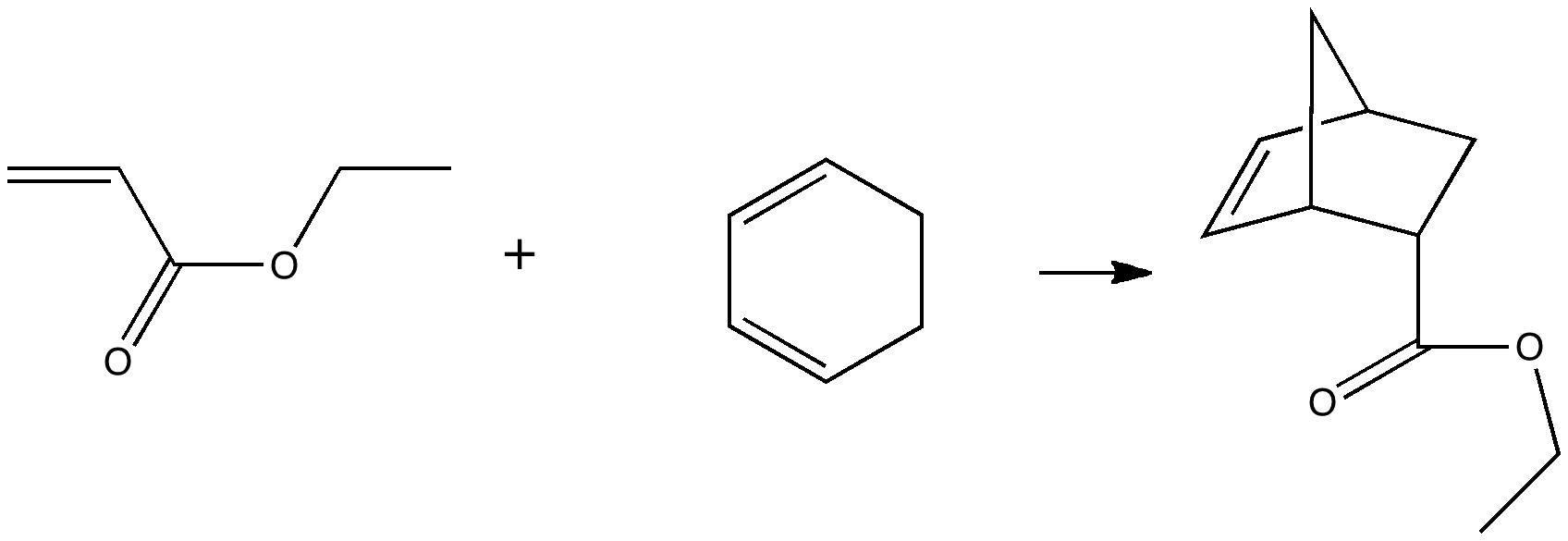
Reactions in cavities can adopt quite different characteristics from those in solvents. Thus first example of the catalysis of the Diels-Alder reaction inside an organic scaffold was reported by Endo, Koike, Sawaki, Hayashida, Masuda, and Aoyama[cite]10.1021/ja964198s[/cite], where the reaction shown below is speeded up very greatly in the presence of a crystalline lattice of the anthracene derivative shown below. A Diels-Alder reaction.

Cavities promote reactions, and they can also trap the products of reactions. Such (supramolecular) chemistry is used to provide models for how enzymes work, but it also allows un-natural reactions to be undertaken.
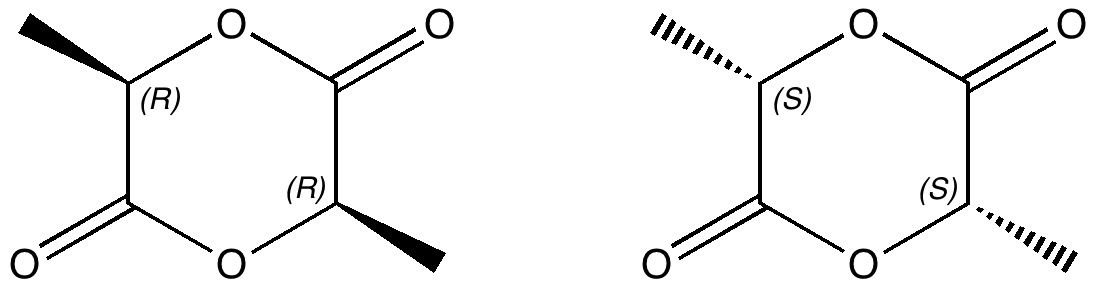
Lactide is a small molecule made from lactic acid, which is itself available in large quantities by harvesting plants rather than drilling for oil. Lactide can be turned into polymers with remarkable properties, which in turn degrade down easily back to lactic acid. A perfect bio-renewable material!
