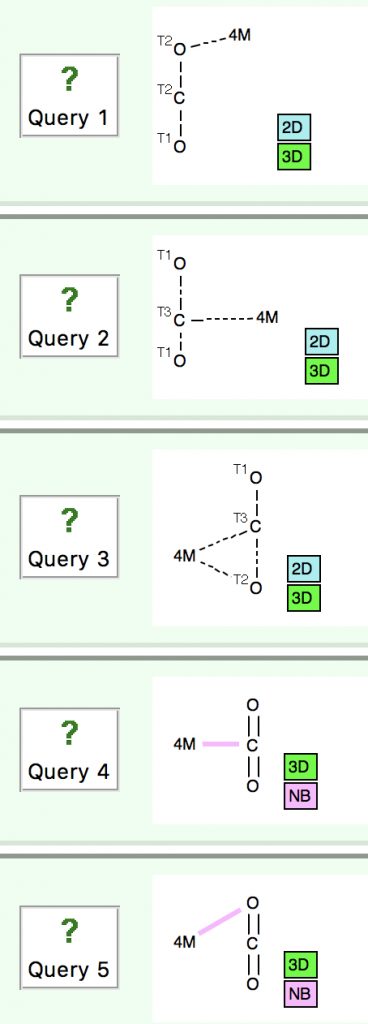
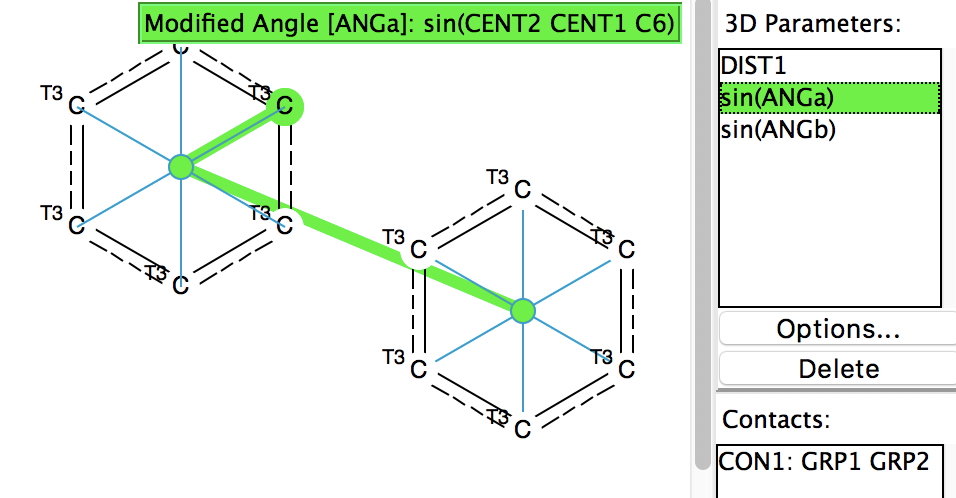
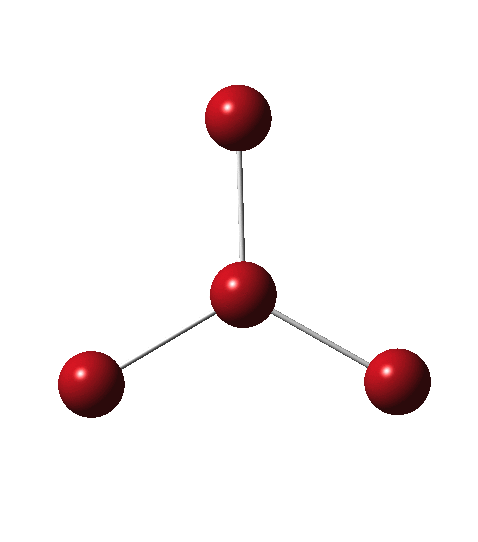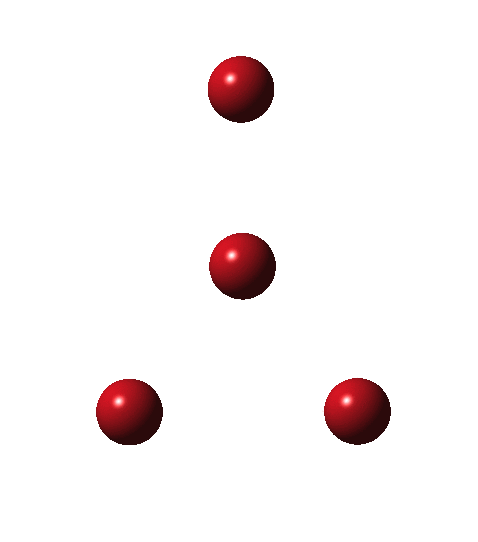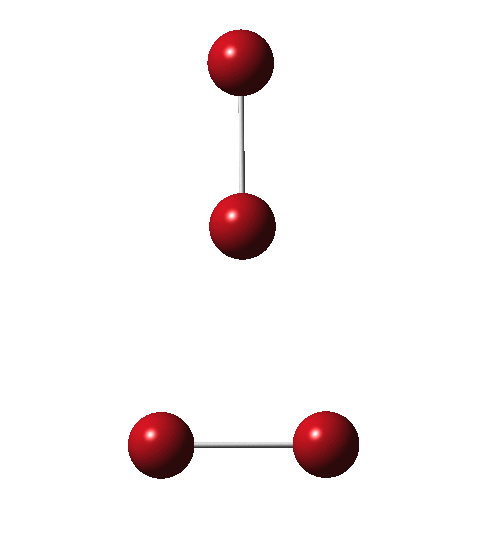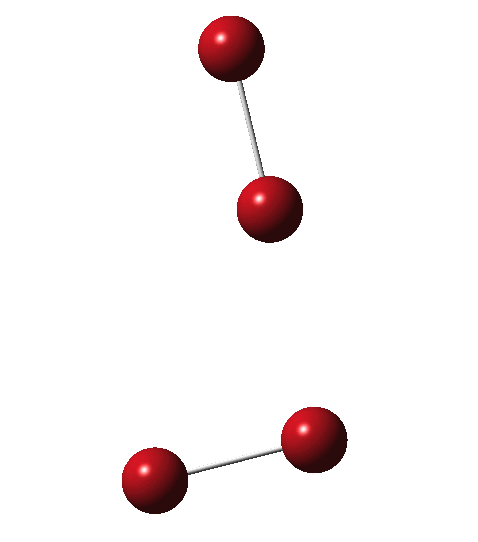
Following the general recognition of carbon as being tetrahedrally tetravalent in 1869 (Paterno) and 1874 (Van’t Hoff and Le Bell), an early seminal exploitation of this to the conformation of cyclohexane was by Hermann Sachse in 1890.[cite]10.1002/cber.189002301216 [/cite] This was verified when the Braggs in 1913[cite]10.1098/rspa.1913.0084[/cite], followed by an oft-cited article by Mohr in 1918,[cite]10.1002/prac.19180980123[/cite]