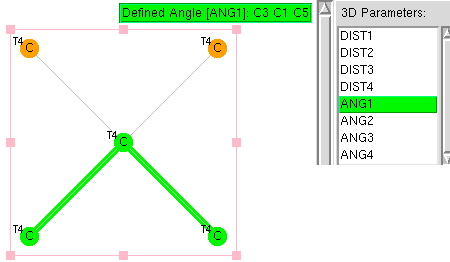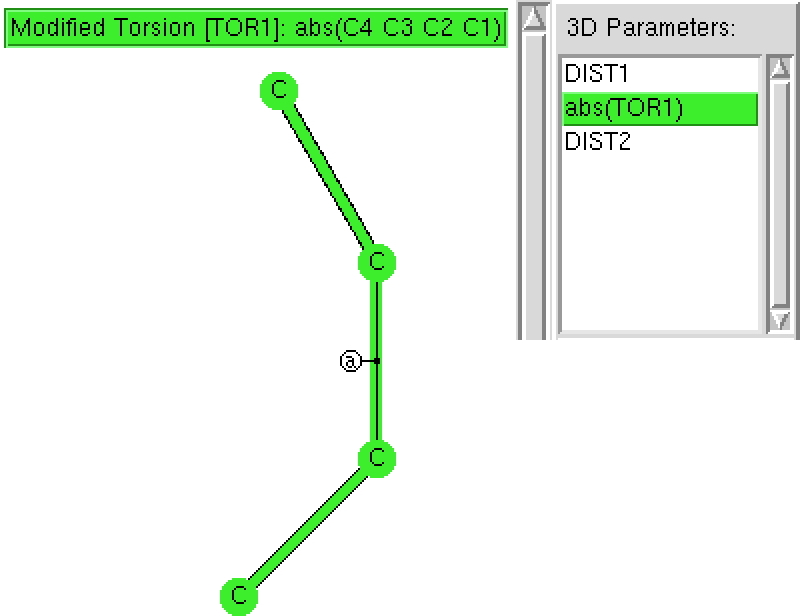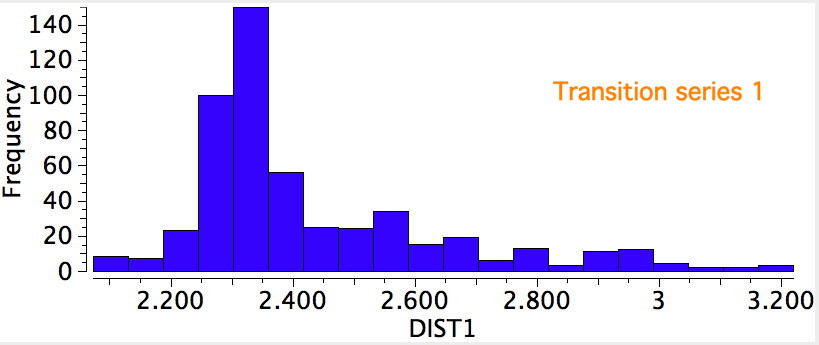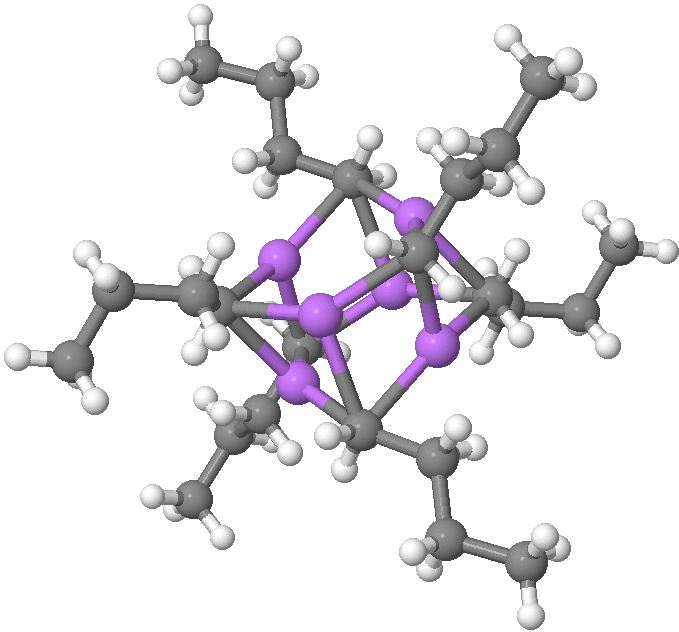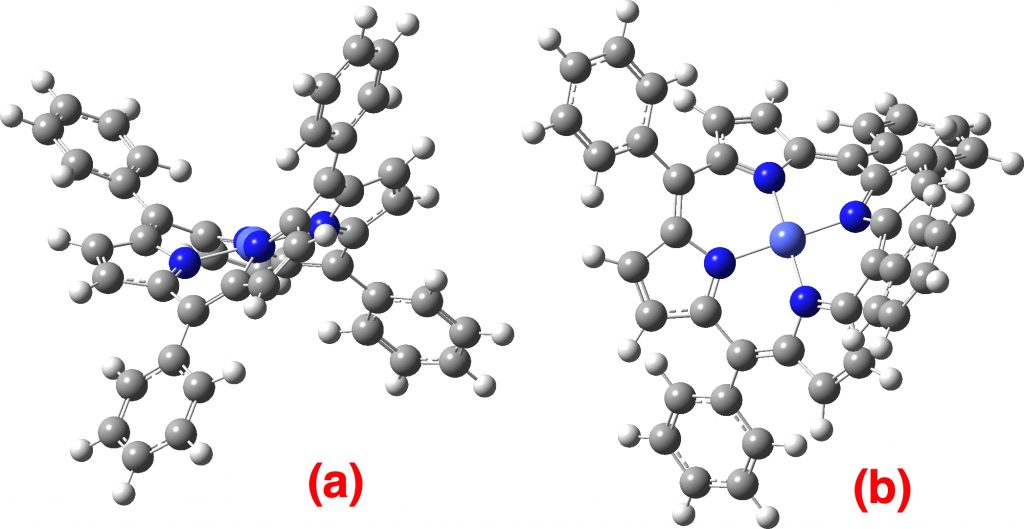
The topic of this post originates from a recent article which is attracting much attention.[cite]10.1038/s41586-019-1059-9[/cite] The technique uses confined light to both increase the spatial resolution by around three orders of magnitude and also to amplify the signal from individual molecules to the point it can be recorded. To me, Figure 3 in this article summarises it nicely (caption: visualization of vibrational normal modes ).