A little while ago I pondered allotropic bromine, or Br(Br) 3 . But this is a far wackier report[cite]10.1126/science.aao7293[/cite] of a molecule of light. The preparation and detection of dimer and trimer bound photon states is pure physics; probably considered by the physicists themselves as NOT chemistry. It is certainly true, as a chemist, that I understood only a little of the article.
Published
Author Henry Rzepa
Published
Author Henry Rzepa

I analysed the bonding in chlorine trifluoride a few years back in terms of VSEPR theory. I noticed that several searches on this topic which led people to this post also included a query about the differences between it and the bromine analogue. For those who posed this question, here is an equivalent analysis.
Published
Author Henry Rzepa
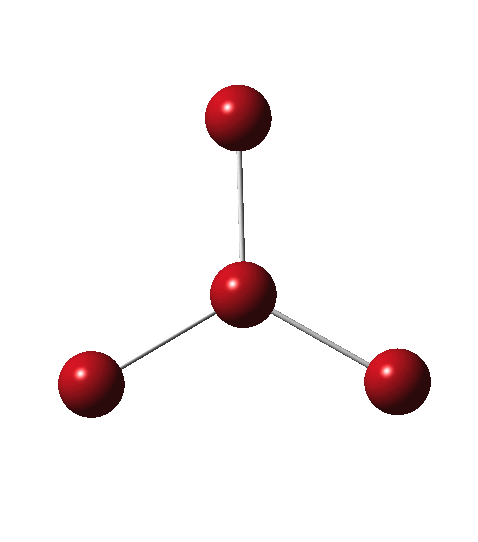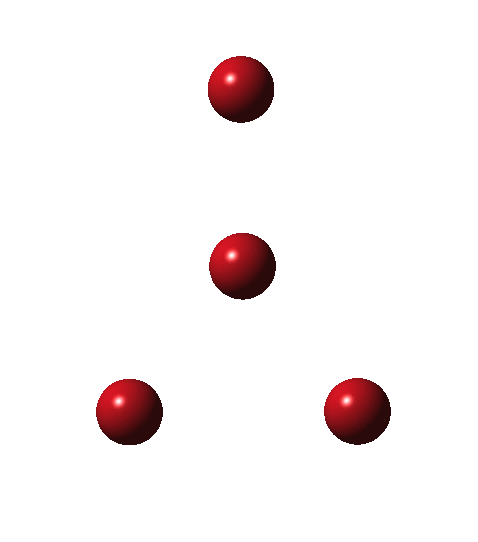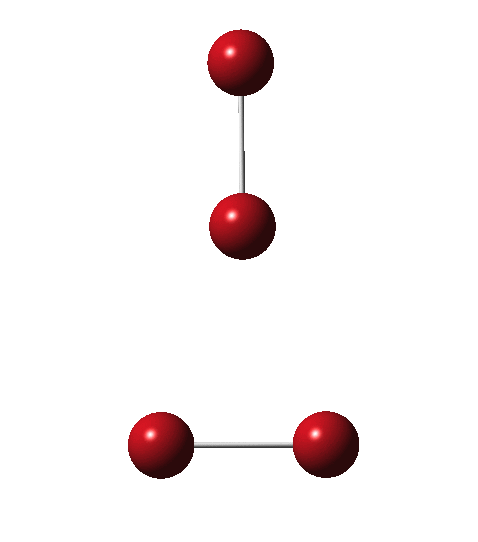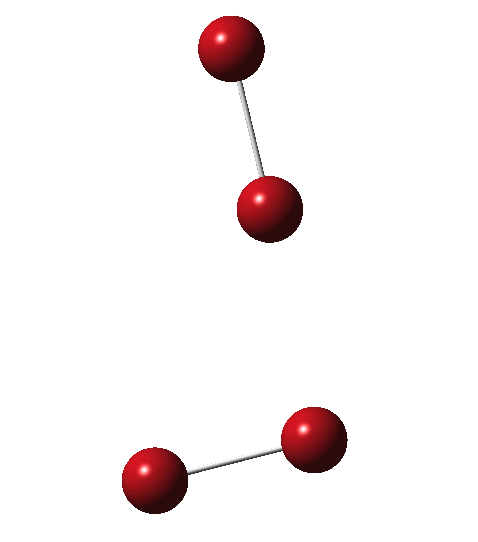
Allotropes are differing structural forms of the elements. The best known example is that of carbon, which comes as diamond and graphite, along with the relatively recently discovered fullerenes and now graphenes. Here I ponder whether any of the halogens can have allotropes. Firstly, I am not aware of much discussion on the topic.
