A few years back I followed a train of thought here which ended with hexacoordinate carbon, then a hypothesis rather than a demonstrated reality. That reality was recently confirmed via a crystal structure, DOI:10.5517/CCDC.CSD.CC1M71QM[cite]10.1002/anie.201608795[/cite]. Here is a similar proposal for penta-coordinate nitrogen. First, a search of the CSD (Cambridge structure database) for such nitrogen.
Semibullvalene is a molecule which undergoes a facile [3,3] sigmatropic shift. So facile that it appears this equilibrium can be frozen out at the transition state if suitable substituents are used. This is a six-electron process, which leads to one of those homologous questions; what happens with ten electrons? A 5,5 double Möbius sigmatropic rearrangement. Click for 3D model.
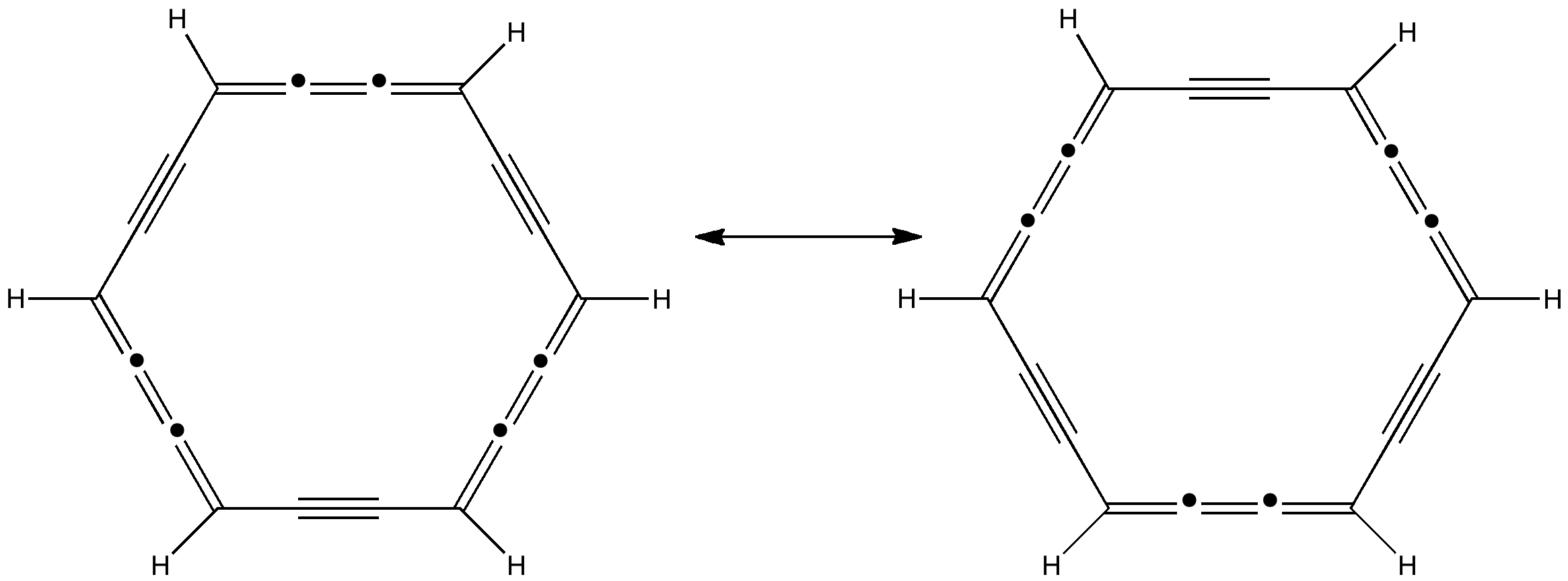
Some molecules, when you first see them, just intrigue. So it was with carbobenzene, the synthesis of a derivative of which was recently achieved by Remi Chauvin and co-workers (DOI: 10.1002/chem.200601193). Two additional carbon atoms have been inserted into each of the six C-C bonds in benzene. Carbobenzene The structure shows two resonance forms, which remind one of Kekulé and of course benzene itself.
