Published
Author Henry Rzepa
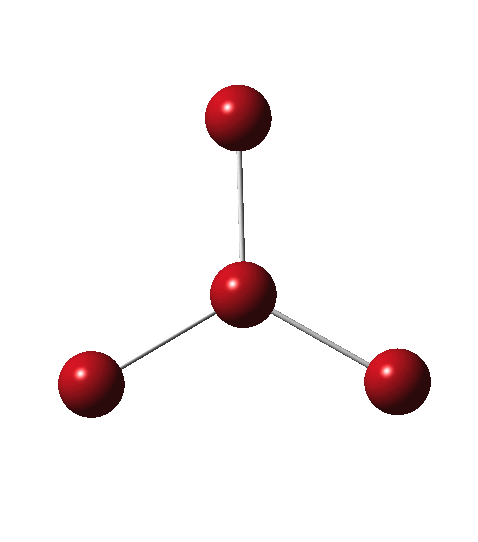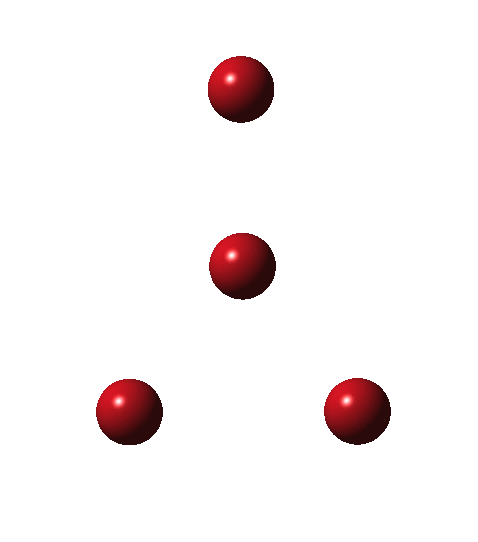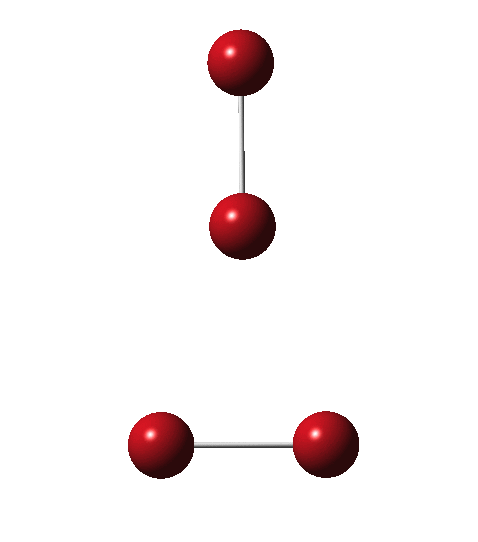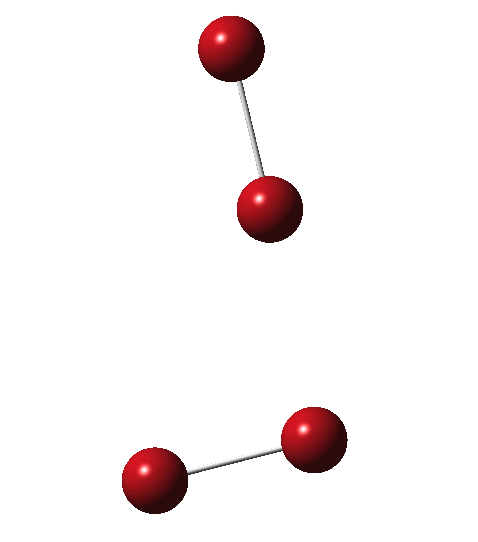
Allotropes are differing structural forms of the elements. The best known example is that of carbon, which comes as diamond and graphite, along with the relatively recently discovered fullerenes and now graphenes. Here I ponder whether any of the halogens can have allotropes. Firstly, I am not aware of much discussion on the topic.
