White City is a small area in west london created as an exhibition site in 1908, morphing over the years into an Olympic games venue, a greyhound track, the home nearby of the BBC (British Broadcasting Corporation) and most recently the new western campus for Imperial College London. ♣ The first Imperial department to move into the MSRH (Molecular Sciences Research Hub) building is chemistry.
My previous dissection of the mechanism for ester hydrolysis dealt with the acyl-oxygen cleavage route (red bond). There is a much rarer[cite]10.1039/jr9550001522[/cite] alternative: alkyl-oxygen cleavage (green bond) which I now place under the microscope.
If you have not previously visited, take a look at Nick Greeves’ ChemTube3D , an ever-expanding gallery of reactions and their mechanisms. The 3D is because all molecules are offered with X, Y and z coordinates. You also get arrow pushing ‡ in 3D. Here, I argue that we should adopt Einstein, and go to the space-time continuum!
HCl reacting with a carbonyl compound (say formaldehyde) sounds pretty simple. But often the simpler a thing looks, the more subtle it is under the skin. And this little reaction is actually my prelude to the next post. The mechanism is studied using ωB97XD/6-311G(d,p) with a simulated solvent (acetic acid) included (but not explicit solvent setting up any hydrogen bonds). Transition state HCl + H2C=O. Click for 3D animation.
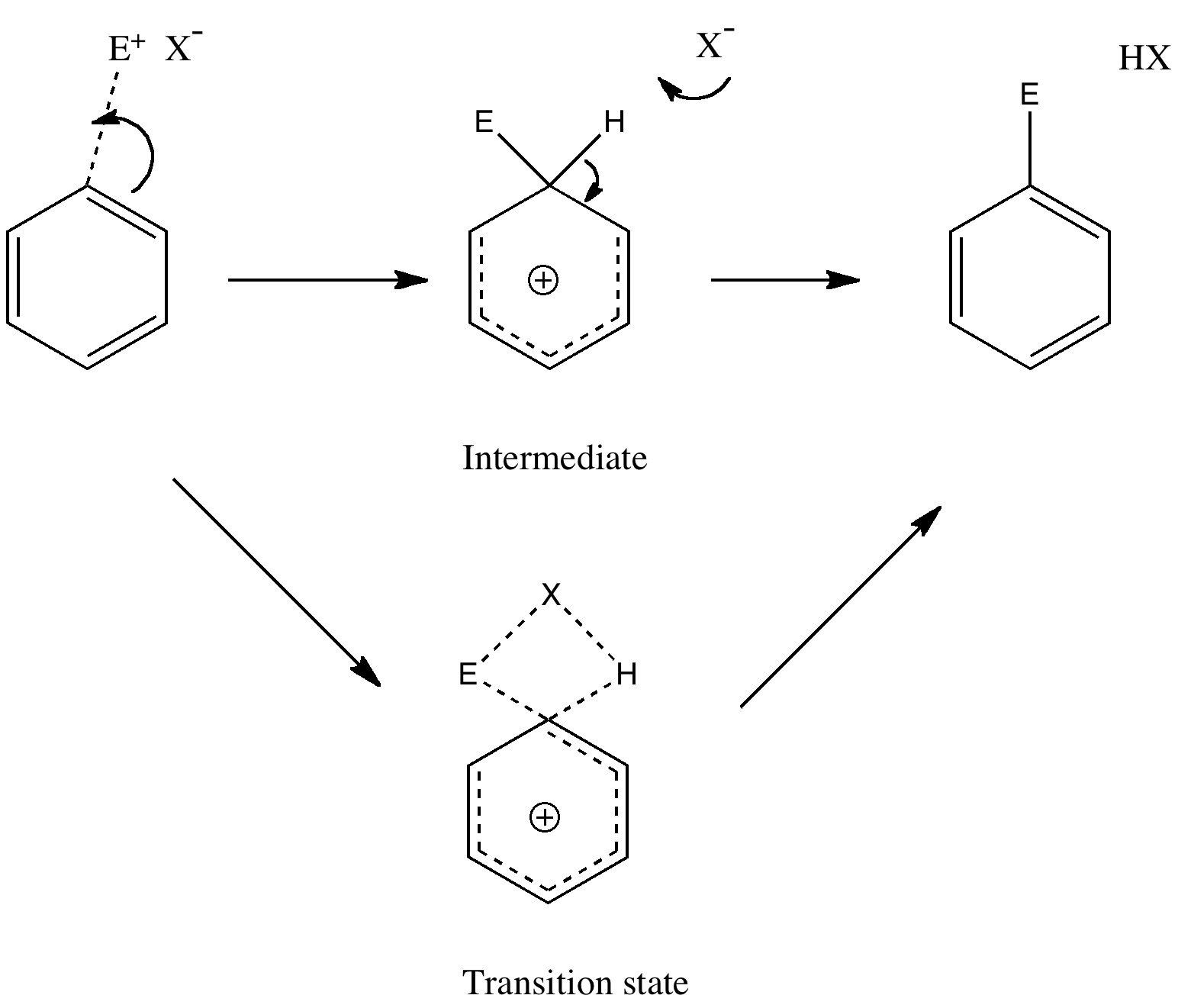
Unravelling reaction mechanisms is thought to be a 20th century phenomenon, coincident more or less with the development of electronic theories of chemistry. Hence electronic arrow pushing as a term. But here I argue that the true origin of this immensely powerful technique in chemistry goes back to the 19th century.
