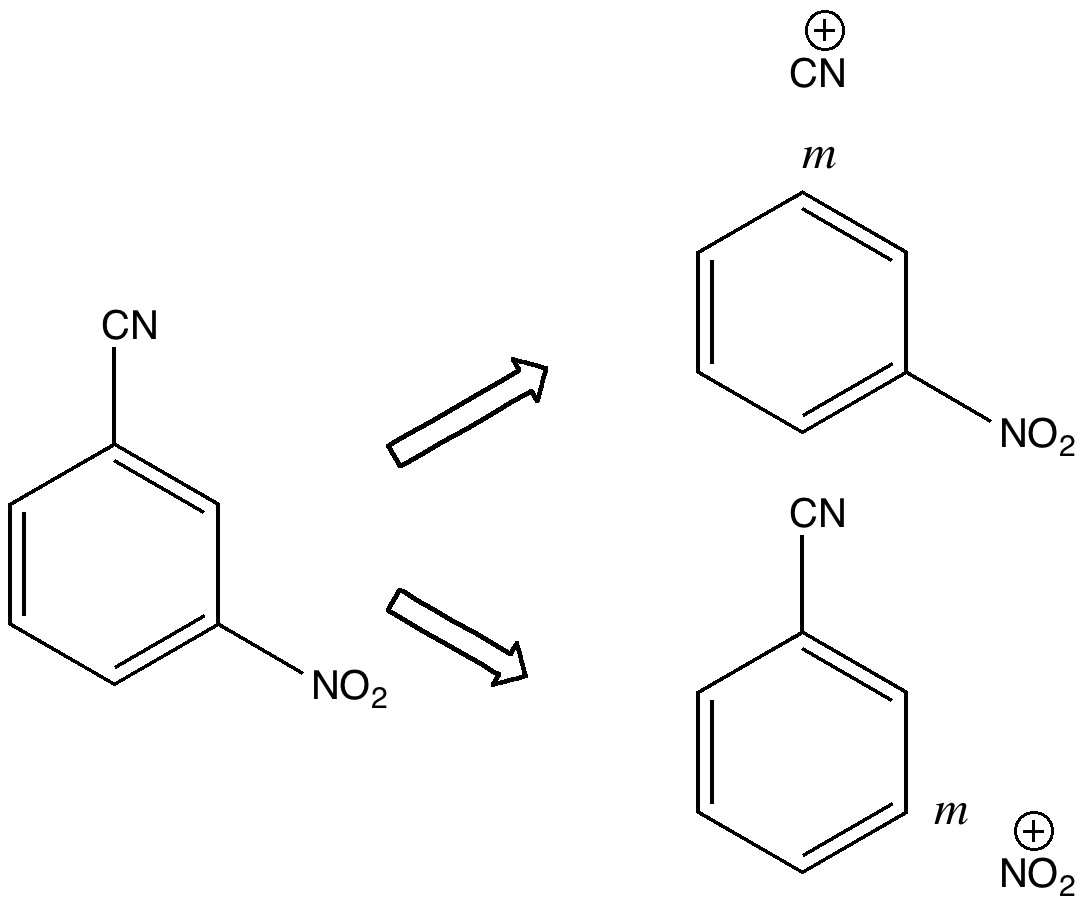
In 2010 I recounted the story of an organic chemistry tutorial, in which I asked the students the question “ how would you synthesize 3-nitrobenzonitrile “. The expected answer was to generate a nitronium ion to nitrate benzonitrile, but can one invert this by generating a C⩸N + ion to cyanate nitrobenzene?